What is geometric isomerism (cis-trans isomerism)?
This type of stereoisomerism occurs when a molecule has two identical groups attached to the same carbon, which leads to different spatial orientations around a double bond.
What is the key difference between structural isomers and stereoisomers?
Structural isomers differ in the connectivity of their atoms, while stereoisomers have the same connectivity but differ in the spatial arrangement of their atoms.
What is the definition of a chiral center?
A carbon atom bonded to four different groups.
What does it mean if a compound is optically active?
A compound is optically active if it can rotate plane-polarized light.
What does the "m/z" value in a mass spectrum represent?
The mass-to-charge ratio of an ion detected by the mass spectrometer.
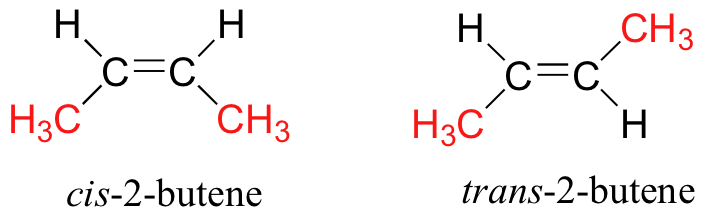
Explain the differences
In cis-2-butene, the two methyl groups are on the same side of the double bond, while in trans-2-butene, they are on opposite sides.
What is the term for stereoisomers that are non-superimposable mirror images of each other?
What is the term for two molecules that are non-superimposable mirror images of each other?
Enantiomers
What type of stereoisomers can exhibit optical activity?
Enantiomers can exhibit optical activity because they are non-superimposable mirror images of each other.
What happens to a molecule during the ionization stage in mass spectrometry?
A molecule is bombarded with high-energy electrons, causing it to lose an electron and form a positively charged molecular ion (M⁺ or M⦁⁺).
Why does 1-butene not exhibit cis-trans isomerism?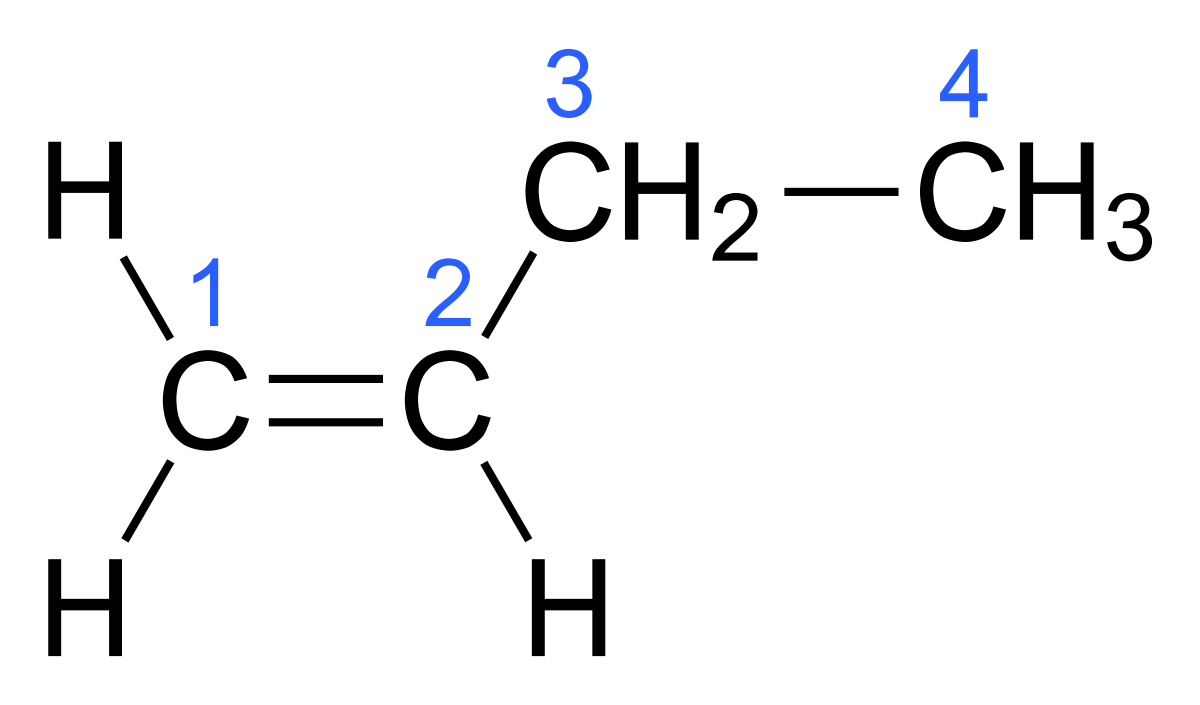
1-butene does not exhibit cis-trans isomerism because one of the carbon atoms in the double bond is bonded to two identical hydrogen atoms, making it impossible to distinguish different spatial arrangements.
What is the main criterion for a molecule to be chiral?
A molecule is chiral if it contains a carbon atom bonded to four different groups, making it non-superimposable on its mirror image.
A molecule has two chiral centers and one plane of symmetry. How many stereoisomers are possible for this molecule, and why?
Only 3 stereoisomers are possible because one of the stereoisomers is a meso compound, which is optically inactive due to the plane of symmetry. This reduces the number of unique stereoisomers from the maximum 2^2 = 4
What is the name of a 1:1 mixture of two enantiomers, and why is it optically inactive?
A racemic mixture is optically inactive because the rotations of plane-polarized light by each enantiomer cancel each other out.
What does the base peak in a mass spectrum represent, and why is it significant?
The base peak represents the most abundant ion detected in the mass spectrometer. It is significant because it provides information about the most stable or frequently formed fragment during ionization.
What is a requirement for substituents around a double bond to exhibit cis-trans isomerism?
Each carbon in the double bond must have two different groups attached to it.
Which of the following compounds are stereoisomers?
A) (R)-2-chlorobutane and (S)-2-chlorobutane
B) 1-bromo-2-chloropropane and 2-chloro-3-bromopropane
C) (E)-but-2-ene and (Z)-but-2-ene
Explanation: A are enantiomers; C are geometric isomers. B is not stereoisomers as they have different connectivity
A molecule has two chiral centers and exists as a single stereoisomer. What structural feature must the molecule have for this to occur, and what is the stereochemical consequence?
The molecule must have an internal plane of symmetry, making it a meso compound. This symmetry causes the chiral centers’ optical activities to cancel out, resulting in the molecule being optically inactive despite having chiral centers.
If a solution of an optically active compound rotates plane-polarized light to the left, what can you infer about the compound and its enantiomeric composition?
The compound contains more of the levorotatory (−-−) enantiomer, which causes the light to rotate to the left.
In the mass spectrum of acetic acid (CH₃COOH), the molecular ion peak is observed at m/z=60, and a fragment peak is seen at m/z=45. What does the fragment peak at m/z=45m/z = 45m/z=45 indicate about the structure of the molecule?
The fragment at m/z=45m/z = 45m/z=45 indicates that a methyl group (CH₃) was lost from the molecular ion (CH₃COOH). This suggests that the remaining cation fragment corresponds to CH2COOH+
A molecule contains a double bond with four different substituents: -CH₃, -Cl, -CH₂CH₃, and -H. Using the Cahn-Ingold-Prelog priority rules, describe the steps needed to assign the correct E or Z configuration.
The double bond has the following substituents:
- CH₃ (methyl group)
- Cl (chlorine atom)
- CH₂CH₃ (ethyl group)
- H (hydrogen atom)
Step 1: Identify Priority for Each Carbon of the Double Bond
First Carbon:
- Substituents: Cl and CH₃.
- Rule: Higher atomic number takes priority.
- Chlorine (atomic number 17) > Carbon (atomic number 6).
- Cl gets higher priority.
Second Carbon:
- Substituents: CH₂CH₃ and H.
- Rule: Higher atomic number takes priority.
- Carbon in CH₂CH₃ (atomic number 6) > Hydrogen (atomic number 1).
- CH₂CH₃ gets higher priority.
Step 2: Determine Spatial Arrangement
- Look at the positions of the highest-priority groups:
- Cl and CH₂CH₃.
- If these groups are on the same side of the double bond, the configuration is Z.
- If these groups are on opposite sides, the configuration is E.
Step 3: Assign Configuration
Assume the molecule's geometry:
- If Cl and CH₂CH₃ are opposite: E configuration.
- If they are on the same side: Z configuration.
Final Answer:
The configuration depends on the spatial arrangement:
- If Cl and CH₂CH₃ are on opposite sides: E.
- If Cl and CH₂CH₃ are on the same side: Z.
How many chiral carbons does cholesterol have?

8 chiral carbons: C3, C8, C9, C10, C13, C14, C17, C20
A molecule with the formula C₄H₆Cl₂ contains a double bond and two substituents on each carbon of the double bond. Can this molecule exhibit chirality? If not, explain why.
No, this molecule cannot exhibit chirality. While it may have E/Z isomerism due to the restricted rotation of the double bond, chirality requires a carbon atom to be sp³ hybridized and attached to four different groups. The carbons in the double bond are sp² hybridized and bonded to only three groups each, so they cannot be chiral centers.
A pure enantiomer rotates plane-polarized light by +15°. What would be the optical rotation if the enantiomer is mixed in a 3:1 ratio with its mirror image?
Net Rotation:
Specific rotation of pure enantiomer x (excess of one enantiomer/total amount of both enantiomers)
The mixture is partially racemic. The net rotation would be +15 degrees x (3-1)/(3+1) =+7.5 deg
Why do some elements, such as chlorine or bromine, produce multiple molecular ion peaks in a mass spectrum, and how would this appear for a compound containing chlorine?
Elements like chlorine produce multiple molecular ion peaks because they have isotopes with significant natural abundances. For chlorine:
Cl-35 and Cl-37 result in two peaks in a 3:1 ratio
For a compound containing chlorine, you would observe a pair of molecular ion peaks separated by 2 m/z units, reflecting the isotopes Cl-35 and Cl-37