The formula for iron (III) oxide is . . .
Fe2O3
Number of moles in 49.25g of gold
0.25 moles.
lower activation energy
What is the percent composition of calcium chloride?
Calcium - 36.0%
Chlorine - 64.0%
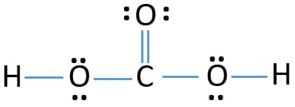
H2CO3 is called . . .
carbonic acid
Write the balanced equation for when hydrogen and oxygen gas combine.
2 H2 + O2 --> 2 H2O
The number of mole ratios possible with the following equation.
AlCl3 + Na2O --> NaCl + Al2O3
six
The three parts of collision theory are . . .
Molecules must collide.
Molecules must collide with the correct orientation.
Molecules must collide with enough energy.
Calculate the empirical formula of a compound that is 43.7% P and 56.3%O by mass.
P2O5
The type of bond formed when electrons create a slightly polar charge on one side of a molecule for brief period of time.
London dispersion
Compound produced from the combination of calcium and aluminum phosphate.
Number of moles of water needed for each mole of each mole of calcium.
Ca + 2H2O --> Ca(OH)2 + H2
1 moles of Ca needed for each 2 mole of H2O
What forms at the top of the graph?
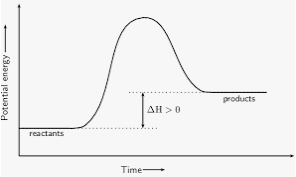
Activated complex
Find the molecular formula of a compound that is 80.0% C and 20.0% H by mass and has a molecular mass of 30.0grams.
C2H6
The name for these three substances.
SO2
H2S
K2S
sulfur dioxide
hydrosulfuric acid
potassium sulfide
Balanced equation for the combustion of octane, C8H18
2 C8H18 + 25 O2 --> 16 CO2 + 18 H2O
Number of moles of lithium oxide needed to react with magnesium chloride to create 120g of the product with magnesium.
Li2O + MgCl2 --> 2 LiCl + MgO
3.0 mol Li2O
What type of reaction occurs in this graph?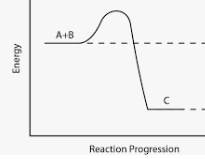
Exothermic
1.5 mol of ethane (C2H6) combine with 4.0 mol of oxygen in a combustion reaction.
Determine the limiting reactant.
Oxygen is limiting
Balanced equation for the reaction of sodium carbonate and magnesium hydroxide.
Na2CO3 + Mg(OH)2 --> 2 NaOH + MgCO3
How many grams of Na2O are needed to create 118 grams of NaCl?
AlCl3 + Na2O --> NaCl + Al2O3
62.5g Na2O
Identify the type of reaction and use the graph to explain how much energy will be absorbed or released. 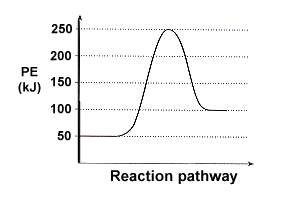
Endothermic : 50 kJ absorbed.
What is the number of excess reactant in grams if 520 grams of oxygen and 670 grams of ethane (C2H6)?
530 grams of ethane remain
Draw the lewis structure for carbonic acid.