Which of the following would be considered matter? Choose all that apply.
A. light from the sun
B. milk in a carton
C. heat from a candle
D. sound of booming thunder
E. pebble on a beach
B. milk in a carton
E. pebble on a beach
What is the smallest particle to retain the properties of an element?
Atom
When two or more of the same type of atom or different types of atoms join together, a __________ is formed.
Molecule
Which of these properties does a typical non-metal element have?
A. It is a good conductor of heat
B. It has a high density
C. It is a poor conductor of electricity
D. It has a low density
C. It is a poor conductor of electricity
A molecule of carbon dioxide is made up of a carbon atom and two oxygen atoms. Which of the following models represents carbon dioxide?
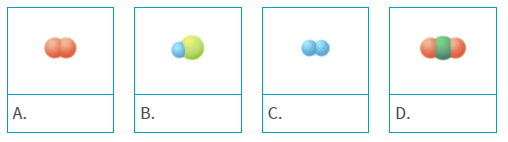
D
What makes physical properties different from chemical properties?
Physical properties relate to elements rather than compounds.
Physical properties appear only after a chemical change occurs.
Physical properties describe elements in the solid state rather than in the liquid or gas state.
Physical properties can be observed without attempting to change the identity of the substance.
4. Physical properties can be observed without attempting to change the identity of the substance.
Chemists use the periodic table to organize the elements. What are some ways in which this organization is useful? Select all that apply.
A. It groups elements that have similar properties.
B. It identifies which elements are most common and which are rare.
C. It allows scientists to predict how a particular element will interact with others.
D. It arranges elements in order of usefulness for human activities.
A. It groups elements that have similar properties.
C. It allows scientists to predict how a particular element will interact with others.
Which of the following can be observed from the model of water? Select all that apply.
A. Water is a molecule.
B. Water is a compound.
C. Water is made up of three types of atoms.

A. Water is a molecule.
B. Water is a compound.
Water is a molecule (two or more atoms held together by chemical bonds). Water is also a compound (the water particle is made up of two or more different types of atoms). A molecule of water consists of three atoms, but only two types of atoms: hydrogen and oxygen.
Which statement about metals and non-metals in the periodic table is true?
A. There are more non-metal elements than metal elements
B. Metals are on the left of the stepped line, and non-metals are on the right of the stepped line
C. Metals are on the right of the stepped line, and non-metals are on the left of the stepped line
B. Metals are on the left of the stepped line, and non-metals are on the right of the stepped line
Which of the following components of air is a compound?
A. argon
B. carbon dioxide
C. nitrogen
D. oxygen
B. carbon dioxide
How are mass and weight similar to, and different from, each other? Select all that apply.
A. They both depend on the amount of matter in a substance.
B. They are both the same for a given object on Earth and on the Moon.
C. The only way to change them is to add or remove matter from an object.
D. Weight is measured in newtons and mass is measured in grams.
A. They both depend on the amount of matter in a substance.
D. Weight is measured in newtons and mass is measured in grams.
Which of these is a way in which elements and compounds are similar?
A. Elements and compounds are both pure substances.
B. Elements and compounds are both listed on the periodic table.
C. Elements and compounds are both made up of different kinds of atoms.
D. Elements and compounds can both be broken down by physical changes.
A. Elements and compounds are both pure substances.
Which image shows a mixture of two compounds?
A
Which of these things you will not find in the periodic table?
A. Element Name and Symbol
B. Atomic Weight
C. Number of neutrons
D. Atomic Number
C. Number of neutrons
The mass of a length of metal wire is 45 g. When the wire is placed in a graduated cylinder with water, it displaces 5 mL of water. What is the density of the metal?
D = m ÷ v
Density is equal to mass divided by volume.
D = m ÷ v
D = 45 g ÷ 5 mL
D = 9 g/ml (or 9 g/cm3)
Which of the following are requirements for something to be a physical property? Choose all that apply.
A. The property can be observed without changing the identity of the substance.
B. The property depends on the amount of the substance present.
C. The state of the substance changes when you measure the property.
D. The property can be observed or measured.
A. The property can be observed without changing the identity of the substance.
D. The property can be observed or measured.
Physical properties are properties that you can observe or measure without changing the substance into a new substance. Some physical properties depend on the amount of a substance present, but others do not.
The diagram shows a portion of the periodic table of elements.
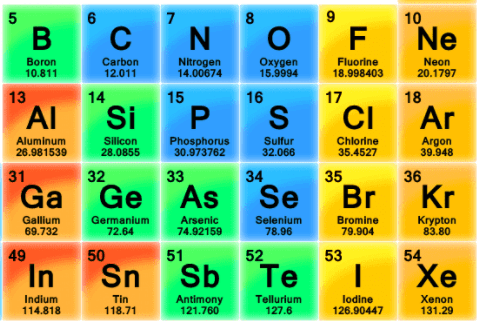
What is the atomic mass of the element silicon?
28.0855
Manganese (Mn) is a silver-colored metal. It reacts with oxygen to form manganese oxide (MnO2), which has two oxygen atoms for every manganese atom. Which statement describes the properties of manganese oxide?
A. It will be a gas because there are more oxygen atoms.
B. It will be a silver-colored metal because manganese is heavier than oxygen.
C. Its properties will be different from manganese and oxygen because it will have some properties of each of the atoms that makes it up.
D. Its properties will be different from manganese and oxygen because the properties depend on the type of atoms and how they are arranged.
D. Its properties will be different from manganese and oxygen because the properties depend on the type of atoms and how they are arranged.
How are the elements of a period arranged in the modern periodic table, from left to right?
A. In order of increasing atomic mass
Diamond is a covalently bonded network solid that is made up of carbon atoms arranged in a way that gives diamonds their hardness. Which of these characteristics do diamonds share with all network solids?
A. low melting point
B. rarity and high cost
C. orderly crystal structure
D. made up of carbon atoms
C. orderly crystal structure
The two beakers shown contain pure water.
Which of these is a chemical property of the water in both beakers?
A. The water is colorless.
B. The water freezes at 0 °C.
C. The water reacts with some metals.
D. The water has a total volume of about 400 mL.
C. The water reacts with some metals.
Which pair of elements is most likely to have similar properties?
A. tungsten and osmium
B. osmium and hassium
C. seaborgium and rhenium
D. bohrium and tungsten
/PeriodicTableWallpaper-56a12d103df78cf7726827e8.png)
Osmium and hassium are in the same group, so they are the most likely to have similar properties.
Ethane is a substance that is used when making plastic. A molecule of ethane is made of two carbon (C) atoms and six hydrogen (H) atoms. The carbon atoms are joined by a chemical bond. Each of the hydrogen atoms is joined to one of the carbon atoms by a chemical bond. Which is an accurate model of ethane?
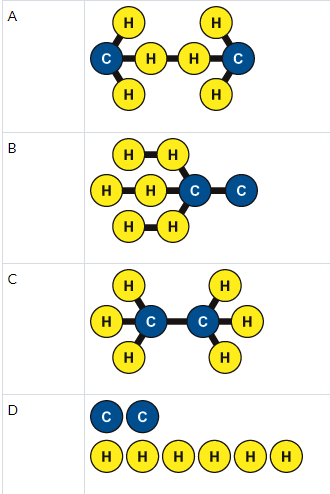
C

Which element is in group 2 and period 3 of the periodic table?
A. Magnesium
B. Boron
C. Calcium
A. Magnesium
A Martian that has spent all of its life on Mars finally makes a trip to Earth. It finds it has a hard time getting around and that objects seem much heavier than on Mars. Why is this?
A. Mars has less mass than Earth, which gives it less gravity, so objects weigh less on Mars.
B. Mars has less mass than Earth, which gives it less gravity, so objects have less mass on Mars.
C. In space, objects have no weight, so the Martian lost strength while traveling, and objects seem heavier.
D. The atmosphere is thicker on Earth than on Mars, which weighs down objects more and makes it harder to move them around.
A. Mars has less mass than Earth, which gives it less gravity, so objects weigh less on Mars.