These are present in foods and give them a sour taste.
What are acids?
These are present in many foods and give them a bitter taste.
What are bases?
Fruit Salad is an example of this.
What is a heterogenous mixture?
A substance that only contains one type of atom.
What is an element?
TRUE OR FALSE: When acids and bases are mixed together, they can neutralize one another.
What is TRUE?
These are used in many cleaners.
What are bases?
"Reacting with metals to produce hydrogen gas" is a property of this.
What are acids?
Milk is an example of this.
What is a colloid?
A solution that contains as much substance as it could possibly hold.
What is a saturated solution?
Which of the following is the strongest base: Coffee, Tomato Juice, baking soda, vinegar.
What is baking soda?
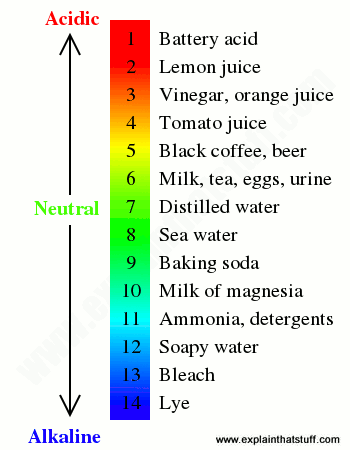
According to the pH scale shown, this substance is the most basic.
What is Lye?
According to the pH scale below, how many more times acidic is lemon juice than milk?
What is 10,000 times more acidic?
Diamonds are examples of this.
What are elements?
A mixture where the parts are evenly dispersed and cannot be seen.
What is a homogenous mixture?
You are making hot chocolate by mixing hot chocolate powder into warm milk. The milk in this situation is this part of a solution.
What is the solvent?
According to the pH scale shown, these substances are more acidic than soapy water, but more basic than milk of magnesia.
What are ammonia and soapy water?
This is what happens when a base is dissolved in water; it causes an indicator to change color.
What is the base reacts with the water and forms hydroxide ions, which cause the indicator to change color?
Molecules of carbon dioxide re examples of this.
What is a compound?
A solution that has a high concentration of hydroxide ions.
What is a base?
The concentration of a solution with 7g of sugar dissolved in 0.5L of solution.
What is 14 g/L?
What is [H3O+] > [OH-]?
"Being corrosive to skin" is a property of this material.
What are acids AND bases?
A material that can be physically separated into different components is known as this.
What is a mixture?
The part of a solution that is dissolved in another part.
What is the solute?
Two factors that can change the solubility of a substance.
What are pressure and temperature?