This copper wire would be classified as a(n) ______.

Element
True or false: Melting butter is a chemical reaction
False
It's still the same stuff. Just a different form of it.
A ______ solution contains the maximum amount of solute dissolved within a specific amount of solvent at a given temperature and pressure.
Saturated
How many protons does a helium atom have?

2
What college did Polk go to?
Carleton College
Carbon dioxide would be categorized as a(n) _____.
Compound (two or more elements bonded together)
Which phase/state of matter takes the shape, but not the volume of its container?
Liquids
"Likes dissolves likes" tells us that _______
Polar solutes can dissolve in polar solvents.
Non-polar solutes can dissolve in non-polar solvents.
What are the two subatomic particles inside of the atom called?
** Bonus 50 points: What is the center of the atom called?
Proton and neutron
** Nucleus
What is one of Polk's two favorite super heroes?
** Bonus 50 if you get both.
Batman and Black Panther
Classify the following as heterogenous mixtures or homogeneous mixtures.
A. 
B. :max_bytes(150000):strip_icc()/raspberry-lemonade-20ea9d581e294c088fdcdfce22f562bb.jpg)
C. 
A - Heterogeneous
B - Heterogenous
C - Homogeneous
As a solid turns to a liquid, what happens to its thermal energy?
Increases
What are the two parts of every solution?
Solute (the thing that gets dissolved)
Solvent (usually water)
How many electrons does Mg2+ have?
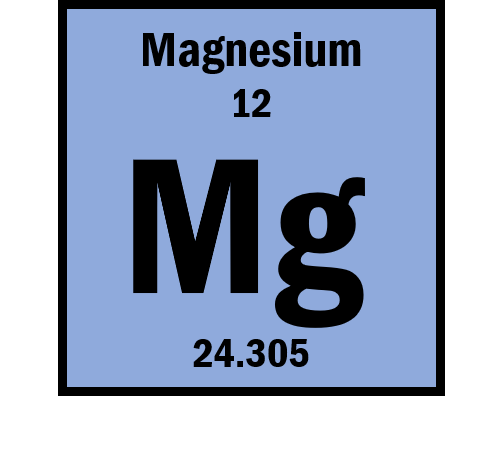
10
** There are 12 protons. 12-10=+2
** Protons - Electrons = Charge
How many tattoos AND how many siblings does Polk have?
Four and Four (150 points per)
Matter is something that has ____ and ______ ______
Has mass and occupies space
Which phase(s) could we not compress?
Solid
Name three of the four ways that you could increase how fast/how well a solid substance dissolves.
Agitation (stir, shake, mix)
Increase temperature
Particle size/surface area (crush it)
Add more solvent (usually water)
What is the term for atoms with the same atomic number, but different masses?
Isotope
(Like Carbon-12, Carbon-13, and Carbon-14)
Put these in order of Polk's favorite way to spend a calm summer day (favorite way first).
A. Paddle boarding
B. Going to a museum
C. Reading at a lake
D. Watching a movie
C. Reading at a lake
A. Paddle boarding
B. Museum
D. Movie
Steel would be classified as a(n) _____.
Mixture (specifically an alloy)
What is the phase change from a solid directly to a gas.
Bonus: What is it called when a gas goes directly to a solid?
Solid --> Gas: Sublimation
Gas --> Solid: Deposition
Name at least one technique used to separate mixtures.
** Each additional technique is 50 points extra. Can only name three.
Distillation - Separation by boiling point
Filtration - Separation by size/phase (bigger pieces or solids stay behind)
Chromatography - Separation by polarity
1. What are the two elements that are liquid at standard temperature and pressure?
2. What separates metals and non-metals on the periodic table?
50 point bonus: What is a common element that breaks that trend?
1. Mercury and bromine
2. The staircase (or metalloids)
Bonus: Hydrogen
Which is the lie?
A. I've been on a broadway stage
B. I visited three national parks this summer
C. I wanted to be a doctor growing up
C
- I was on the stage for "Water for Elephants" last year (not performing)
- I visited Wind Cave, Badlands, and Voyageurs National Parks this summer
- I wanted to be an astronaut growing up