These are the three subatomic particles.
What are protons, neutrons, and electrons?
The total number of electrons in a neutral Chromium atom.
What is 24?
The most reactive group of metals on the periodic table.
What are the alkali metals (or Group 1 or Group IA)?
The trend in atomic radius as you move across a period.
What is decreasing atomic radius?
The type of chemical bonding seen in the following image:

What is ionic bonding?
Located inside of the nucleus, determine the identity of the atom and have a positive charge.
What are protons?
The 3 rules of electron configuration.
What are Hund's rule, the Pauli exclusion principle, and the Aufbau principle?
The range of group numbers that includes the transition metals.
What are groups 3-12?
The following elements, in order of increasing atomic size: Rb, Cd, Xe, Zr
What is Xe, Cd, Zr, Rb?
The type of chemical bonding best represented by this image:

What is metallic bonding?
An atom with the same number of protons but different number of neutrons.
What is an isotope?
The noble gas configuration for Strontium.
[Kr]5s2
The elements from this list that are non-reactive and have 8 valence electrons: Br, Ne, Cs, Mg, Au, Ba, Ar, I, Kr
What are Ne, Ar, and Kr?
The element on the periodic table with the highest electronegativity.
What is Fluorine (F)?
The number of unshared valence electrons in a compound seen here:

What is 8?
The mass number, atomic number, charge, number of electrons, and name of the following element.
What is 80, 34, -2, 36, and Selenium?
The Lewis dot structure for Sulfur, and what the dots in Lewis diagrams represent.
What is

and valence electrons?
The chemical family name of the "salt-forming" non-metal elements.
What are Halogens?
The following elements in order of decreasing ionization energy: Sn, Rb, Ag, Sb
What is Sb, Sn, Ag, Rb?
The number of covalent bonds observed in this dot structure:
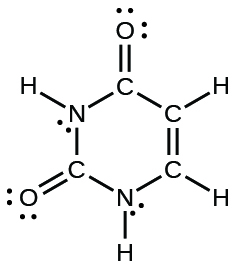
What is 15?
The average atomic mass of element X (rounded to 2 decimal places) AND the actual name of element X.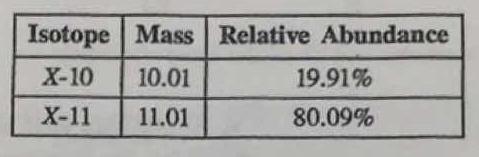
What is 10.81 amu and Boron?
The full electron configuration for Tellurium.
What is 1s22s22p63s23p64s23d104p65s24d105p4?
The element from the following list that does NOT belong: Tungsten (W), Gold (Au), and Indium (In)
What is Indium (In)?
The principle behind why atomic radius increases and ionization energy decreases down a group, based on the positively charged nucleus having less of an attractive pull on the highest energy electrons.
What is nuclear shielding?
The number of Potassium (K) atoms needed to form a stable ionic compound with Nitrogen (N).