What element has a H symbol? ![]()
Hydrogen!
What are the three states of matter? (Must name all three to get full points).
 Solid, liquid and gas
Solid, liquid and gas
What is a mixture?
A mixture is a combination of different substances.
Please draw and show where the electrons and proton are on an atom.

In a __?___ the particles are close together and arranged in a familiar pattern.
Solid
Which of the following methods can be used to sperate a mixture of iron and copper fillings?
A) Magnetic separation
B) Crystallization
C) Evaporation
D) Distillation
A) Magentic Seperation
What number tells you the number of protons and electrons in an element?
The atomic number 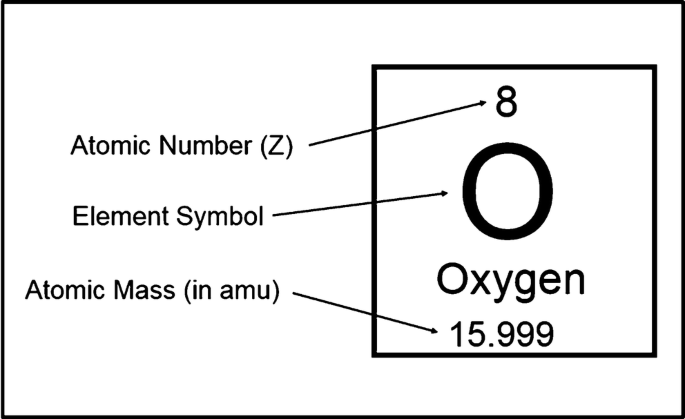
Boiling is the change of state from ___ (100 points) to a ___ (100 points) through the process of ____ (100 points).
Liquid to a gas
Process is called evaporation.
Which separation method would be most effective on tea with loose tea leaves?
Filtering
Example using filter paper.
What are groups (columns) in the periodic table? (200 points)
What are periods (rows) in a periodic table? (200 points)
Groups are the number of electrons in the outer shell.
Periods tell us the number of electrons elements have. E.g. First row is 1 ring of electrons.
Condensation is the change of state from ___ to a ____
What is an example of condensation occurring?
From a gas to a liquid
Example may be water outside of a cold drink bottle, a foggy mirror after a shower and reading glasses fogging up.
What are three separating techniques?
Filtering
Decanting
Distillation
What are valence electrons?
They are electrons in the outermost shell that can be shared with other atoms.
What is the process of sublimation and give an example
Sublimation is the change of state from a solid to a gas without being a liquid.
Example is carbon dioxide or also known as dry ice.

What is the purpose of separating mixtures?
- To remove unwanted particles (1)
- To obtain important substances (1)
- Obtain pure substances (1)