What is the principal quantum number (n) and angular momentum quantum number (l) for the 5d sublevel?
n = 5; l = 2
How many valence electrons are in a neutral atom of chlorine (Cl)?
7
Below is the Lewis structure of the peroxide ion. What is the number of lone electron pairs around the left oxygen?
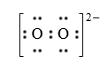
3 pairs
A neutral atom has the following electron configuration: 1s22s22p63s23p1. What is the chemical symbol for the atom?
Al
What subshell from Rb would have to have an electron removed in order to make a +1 cation?
5s
List the following elements in order of decreasing electronegativity (largest to smallest): S, F, O
F > O > S
An anion with a charge of -2 has the following electron configuration: [He] 2s22p6. What the chemical symbol for this ion, including the charge symbol.
O2-
Rank the following elements according to their ionization energy (lowest to highest): beryllium, francium, cesium, rubidium.
Fr < Cs < Rb < Be
Draw the Lewis structure for the phosgene (COCl2) molecule. How many bonding pairs of electrons does the oxygen atom have?
2
A student proposed an electron configuration for an atom containing 28 electrons, which had at least one error. Identify the errors and write the correct electron configuration.
1s22s22p63s23p64s23d8
List the following set of atoms or ions in order of decreasing size (largest to smallest): F, Ne, F-.
F- > Ne > F
A student proposes the following Lewis structure for the isocyanate (NCO−) ion.
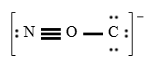
Assign a formal charge to oxygen atom in the student's Lewis structure.
+2
Suppose a popular FM radio station broadcasts radio waves with a frequency of 90. MHz. Calculate the wavelength of these radio waves.
3.3 m
An element has the following electron configuration:
[Ar] 4s23d104p3. Would it be a metal, nonmetal, or metalloid?
metalloid
Draw the Lewis structure for chlorine trifluoride (ClF3). How many electron pairs (bonding and nonbonding) are on the chlorine atom?
5 pairs (expanded octet)