Orbital shaped like a sphere
S-Orbital
The periodic table is arranged by atomic number, which is the number of these
Proton
Due to proton gains, electron repulsion and electron resonance this shrinks down going across the period, but grows going down groups.
Atomic size
This is a particle of light that is massless, and travels in waves
A Photon
Which as largest atomic radius?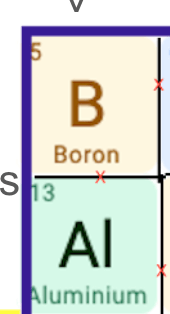
Al
H, He, and groups 1 and 2 end with a specific orbital. We call them the...
s-block
Mars
The p-orbital can hold this many electrons.
6
An atom which has a net charge that is negative or positive is called this.
Ion
Electronegativity and Ionization Energy follow the same trend of what across the period?
Growing across the period
One-half the distance between the nuclei of two atoms of the same element when the atoms are joined.
Atomic radius
Are there 1, 2, 3, or 4 blocks that we have defined in the periodic table?
4 (s-block, p-block, d-block, f-block)
Groups 13-18 (minus He) are apart of this block
p-block
This is the number one selling band in the United States of all time selling 183 million units.
The Beatles
The d-orbital can hold this many electrons
10
Atom or group of atoms with a positive charge.
Cation
Due to electron shielding Electronegativity and Ionization Energy follow the same trend of what down a group?
Getting smaller
The ability of an atom to attract electrons when the atom is in a compound, in other words, the strength of an atom to take an electron.
Electronegativity
Which has greater ionization energy?
P or Ar
Ar
The transition metals are this block
d-block
This cartoon character is described as "He is our hero. Going to bring pollution down to zero."
Captain Planet
The f-orbital can hold this many electrons.
14
Atom or group of atoms with a negative charge.
Anion
Due to the same valence electrons (therefore, having similar repulsion and resonance) this stays constant across a period, and when you add more layers of orbitals grows as it goes down
Electron shielding
The energy required to remove an electron from an atom in its gaseous state, in other words the energy to take away an electron.
Ionization energy
Which has greater electronegativity?
Ca or Ga
Ga
the inner transition metals, often below the periodic table, are this block
f-block
This is the main source of energy for the planet
The Sun
s-orbitals and p-orbitals
Though they don't carry a charge neutrons fill in spaces in protons since protons repel each other. When an element gets a different number of neutrons it is called a what?
Isotope
Why does Fluorine had a higher ionization than Iodine?
Valance electrons have better access to nucleus in Fluorine, less shielding. They are therefore harder to move.
The phenomenon where inner electrons partially block the positive charge of the nucleus.
Electron Shielding
Which has largest atomic radius?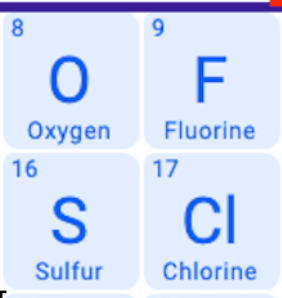
S
There are this many periods in the periodic table
7
Drops of Jupiter