Define valence electrons
The electrons in the outer energy level
As atomic number increases within Group 15 on the Periodic Table, atomic radius_____________
increase
Rows on the periodic table
Periods
The majority of the elements on the periodic table are this.
metals
A subatomic particle with no charge.
a neutron
Define electron configuration
the summary of where the electrons are around a nucleus
What is the group number of noble gases?
18/ 8A
Families in the Periodic Table
Column
What is the formula for finding the neutrons of an atom?
Atomic mass - Atomic number
The location of electrons.
outside the nucleus
Find the element:
1s22s22p63s23p64s23d104p5
Bromine
Arrange the elements in correct order of decreasing electronegativity:
Be, Ba, Ca, Mg, Ra
Be, Mg, Ca, Ba, Ra
How many valence electron does this element have?
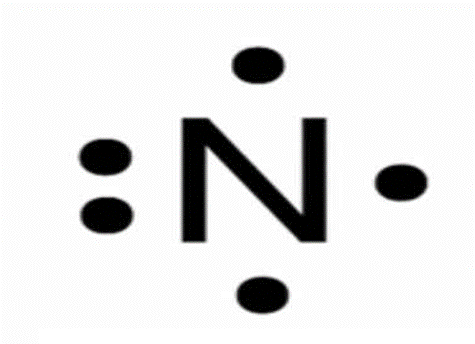
5
What are neutrons of Arsenic (As)
42
Elements in the same group share this.
same number of valence electrons
Predict the element for this configuration:
[Ar]4s23d104p1
31
Ga
Gallium
Represents the relative size of the atoms using the increasing order:
Li, Na, K, and Rb
Li, Na, K, Rb
How many valence electron does Chlorine have?
7
The types of elements found in groups 13-18 include —
nonmetals, semimetals, noble gases, and metals
The three classes of elements are these.
metals, metalloids, and nonmetals
Write the configuration for Na
1s22s22p63s1
Which of these elements has atoms with the largest atomic radius?
Sr, Ba, Rb, Cs
Cs
Elements in group 1 have only 1 valence electron and are considered this.
What are elements that are considered extremely reactive?
Atomic Number and Atomic Mass of Bi
Atomic Number: 33
Atomic Mass: 209
Located on the right side of the zigzag line.
nonmetals