The electrons in the outer energy level
What are valence electrons?
The three classes of elements are these.
What are metals, metalloids, and nonmetals?
The atomic number tells you
What is the number of protons (and electrons) an atom of an element has?
The Element Symbol and Number for Tungsten.
What is W, number 74
A part of an atom with a negative charge
What is an electron?
The modern periodic table is organized according to this.
What are by increasing atomic number?
True/False: Elements in the same group have similar properties.
What is True?
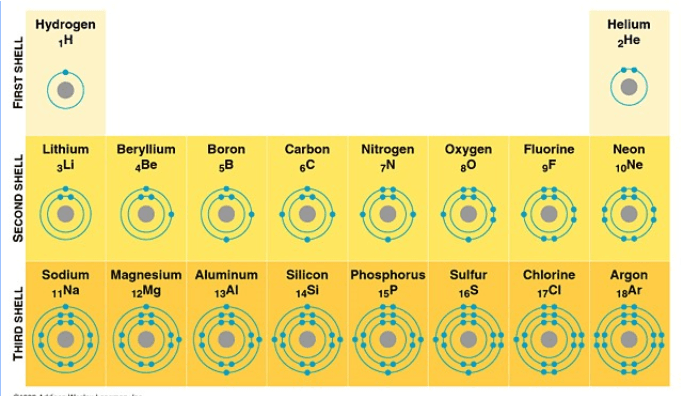
What are Bohr diagrams.
The majority of the elements on the periodic table are this.
What are metals?
The location of electrons.
What is outside the electron cloud?
Columns on a periodic table
What are groups?
Tiny particles that make up all matter.
What are atoms?
This person developed a model of the atom that has the electrons circulating in fixed energy levels around a nucleus.
Who was Neils Bohr?
Most abundant metal on Earth.
What is element 27, Iron?
Elements in the same group share this.
What are the same number of valence electrons
Found in a zigzag line on the periodic table.
What are metalloids?
True/False: Elements in the same group have the same melting point.
What is False?
A part of an atom with a positive charge
What is a proton?
True/False: There are as many types of atoms as there are elements on the Periodic Table.
What is True.
The maximum number of electrons the 1st orbital, or energy shell, can hold.
What is 2?
Rows on a periodic table.
What are Periods?
The part of the atom that tells you its identity.
What is the number of Protons?
A subatomic particle with no charge.
What is a neutron?
The Element Symbol and Number for Arsenic.
What is As, number 33?
The maximum number of electrons the 2nd orbital, or energy shell, can hold.
What is 8?
Located on the right side of the zigzag line.
What are nonmetals?
Atomic mass - Atomic number=
What is the formula for finding the neutrons of an atom?
The location of protons and neutrons.
What is inside the nucleus?
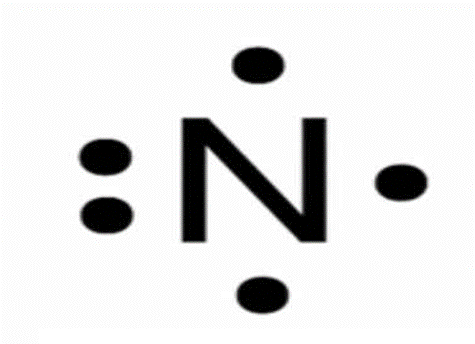
What is dot model for Nitrogen.
The element that will melt in your hand.
What is Gallium?
This person used cards to figure out the pattern of the periodic table.
Who was Mendeleev?
This person thought that the atom was shaped like a ball.
Who was John Dalton?
This person shot Alpha particles through gold foil to discover the nucleus.
Who is Ernest Rutherford?
Elements in group 1 have only 1 valence electron and are considered extremely reactive.
What are Alkali Metals?
A Halogen with 53 electrons.
What is Iodine, element 53?
Groups 3-10 on the Periodic Table.
What are the Transition Elements?
This element is the most abundant in the atmosphere?
What is Nitrogen?
An atom with a completely full outer most (valence) energy level is this.
What is nonreactive or noble gases?
This person named an element but didn't discover it.
Who is "that french guy" Lavoisier?
A group of elements with full outer electron shells.
What are the Noble Gases?