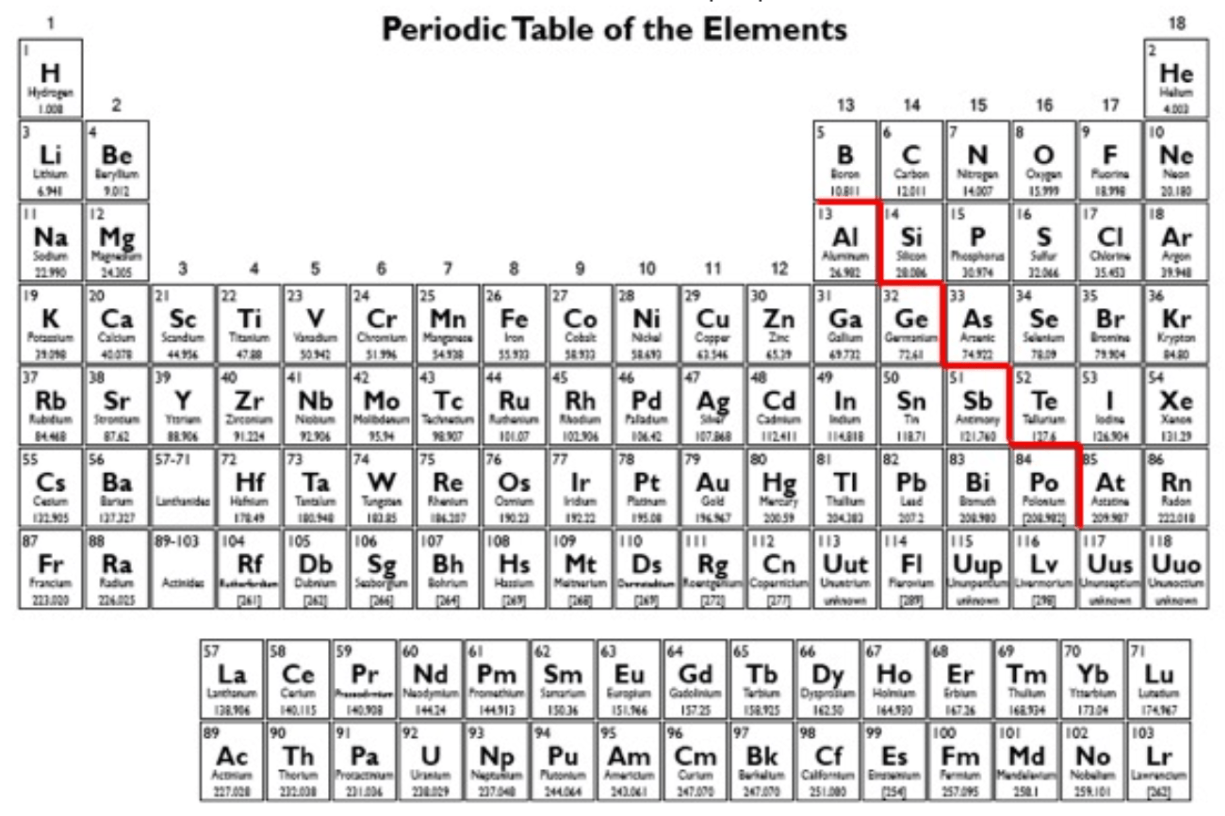
What are the columns in the periodic table called?
What is groups / families?
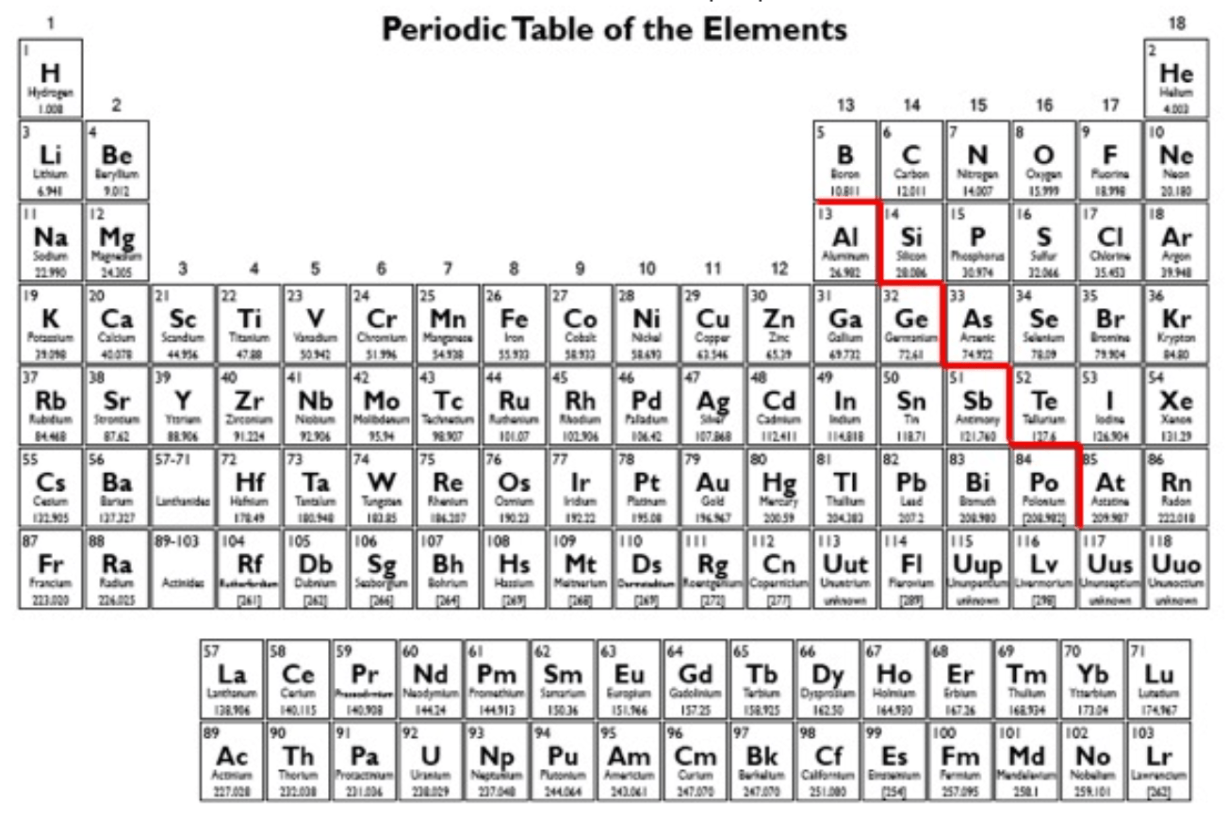
Which has the largest radius among Na, Li and C?
 amu
amu
What is Na
Looking at the periodic table given below, which has the highest reactivity, Mg or Ba?
What is Ba?
The most reactive nonmetal on the perioic table. And which family does it belong to?
What is Fluorine and halogen?
As you go down the group, what happens to the force of attraction between nucleus and the valence electrons and why?
What is decreases because the distance grows
What is the primary reason that the effective nuclear charge felt by the valence electrons decrease down the group?
What is because of the increase distance between the nucleus and the valence electrons.
The unreactive gas used to store alkali metals in, because alkali metals are so reactive (it is in energy level 3).
What is Argon?