Very reactive group 1 metals with only one valence electron in their outer shell
What are alkali metals?
A chemical bond that occurs when one atom "steals" electrons from another atom, causing the charged atoms to then stick together
What is an ionic bond?
When drawing Lewis structures, most atoms need ____ electrons to be stable (unless they are hydrogen, boron, or another exception!)
What is 8 electrons?
Which atom would have a partial negative charge in an HF molecule?
What is fluorine?
Draw the Bohr's model for Hydrogen
Nonreactive elements that have a stable octet of eight electrons in their valence shell
What are noble gases?
Which kind of bond is formed by atoms that share electrons unequally and create partial negative and positive charges that cause it to be attracted to other molecules?
What is polar covalent bond?
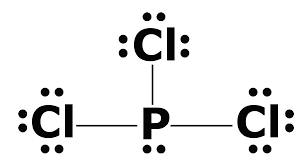
Draw the Lewis Dot Structure for PCl3
C-H bonds are always considered ___________
What is nonpolar?
Draw this bohr's model as a LDS
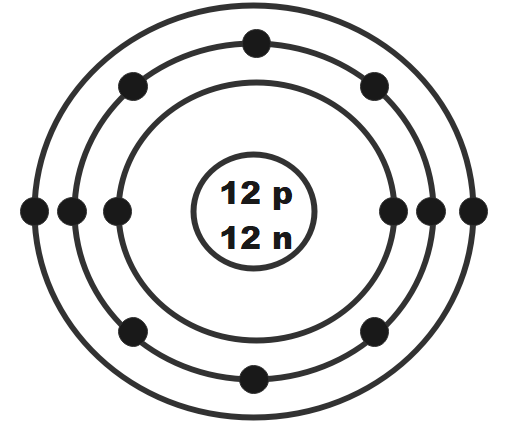
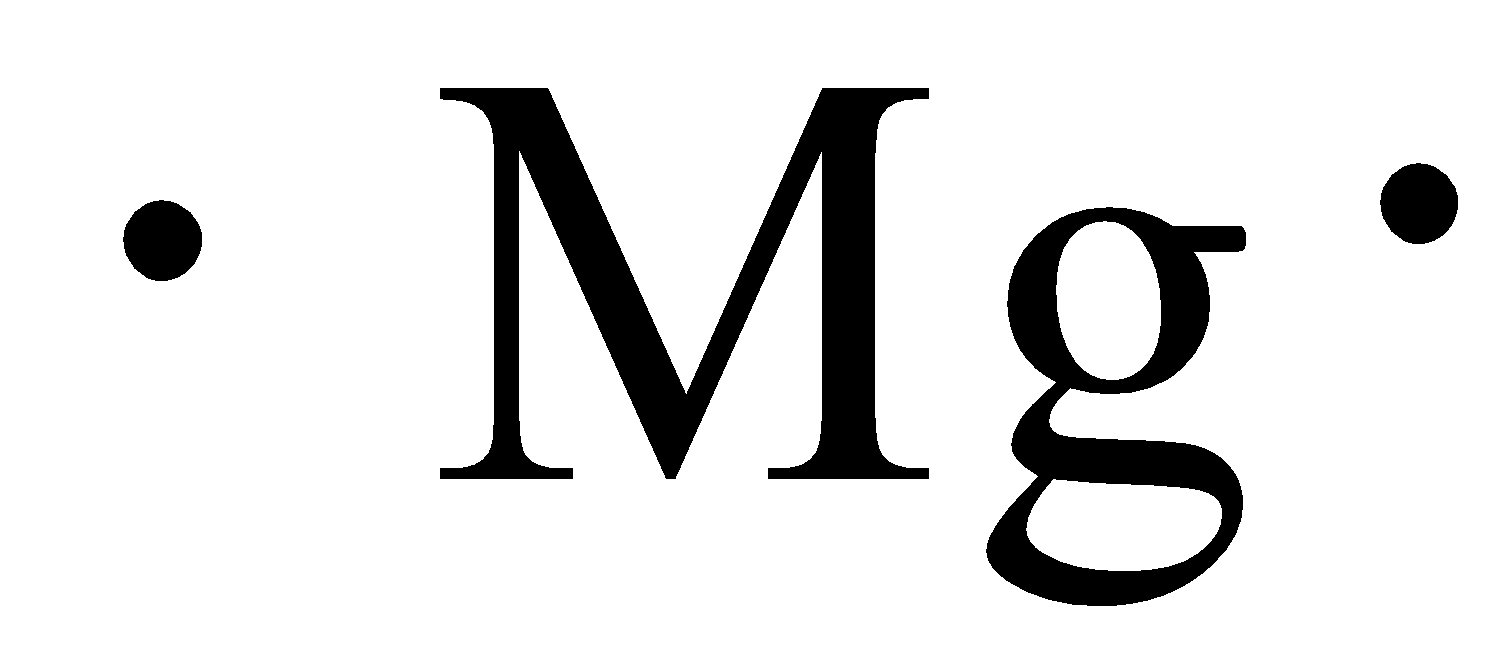
The side on the periodic table that contains most of the nonmetals
What is right side?
Which compound usually has a higher boiling point - ionic or covalent? Explain why.
What is ionic compounds - because they have a crystal lattice made of oppositely-charged ions that stick together strongly; in contrast, covalent molecules are held together by weak intermolecular forces
The formula for a compound formed from Oxygen and Aluminum
What is Al2O3?
This image represents a _____________ bond

What is a metallic bond?
The element represented by the electron configuration: 1s22s22p63s1
What is sodium?
How does electronegativity change as you go across a period from left to right. Explain why.
What is electronegativity increases because the positive charge of the nucleus increases (more protons) and pulls electrons in closer?
Why do most atoms want to bond?
What is atoms want to decrease their potential energy and become stable?
The charge on tin in SnCl4 (write in roman numerals)
What is Tin (IV)?
This range of electronegative difference typically represents a polar covalent bond
The abbreviated electron configuration for silver
What is [Kr]5s24d9?
How does atomic radius change as you go down a group. Explain why.
What is the atomic radius increases because more energy levels are added, and the electrons get farther away from the nucleus - causing the atom to get bigger?
A bond formed between 2 non-metals
What is a covalent bond?
Draw the Lewis structure for C2H6.
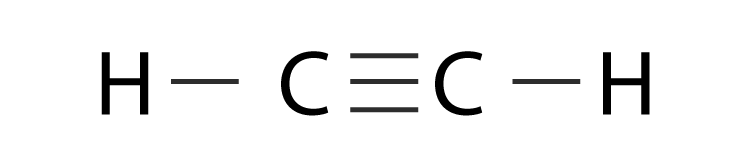
CO is a polar/nonpolar bond (show your work)
The full electron configuration for rubidium
What is 1s22s22p63s23p64s23d104p65s1