Which type of heat transfer happens when you burn your hand on the stove?
What is conduction?
The average kinetic energy of the particles that make up a material.
What is temperature
Material through which thermal, sound, and electrical energy flows easily.
What are conductors
You stay too long in a tanning bed and your skin gets burned. What kind of heat transfer is this?
What is radiation
A friend tells you that insulation keeps out the cold. Explain why this statement is incorrect.
Heat is transferred from hot to cold. So insulation actually keeps the warm in, not the cold out.
How do molecules move in solids?
What is: vibrate in place
Styrofoam prevents outside air transferring energy to the fish inside the cooler because it is an __________
What is Styrofoam is an insulator
Radiation is...
heat transfer through electromagnetic waves
Your hand feels cold when you place it on the lab bench. WHY?
What is your hand is losing thermal energy heat because it is transferring into the lab bench
You are supposed to get low toward the floor if you are ever caught in a building or house fire. The hotter air and smoke will rise and the cooler air will sink. What type of heat transfer does this represent?
What is Convection?
You conduct an investigation comparing the cooling rates of a container of 100 C water and a container of 50 C water. Name two control variables you would use.
What are water volume, size of container, time, environment temp, etc.
Styrofoam prevents the transfer of energy because it is an ___________
What is an insulator?
A bowl of hot soup cools less than a small mug of of the same temperature soup in thirty minutes.
True or False AND explain WHY
Or what is true
the bowl of soup is larger than the mug and contains more thermal energy.
The Three ways thermal energy is transferred are...
What is Radiation, Conduction, Convection
Heat flows from
warmer to cooler temperature
Heat rising and cooling to form currents in a fluid
What is convection?
Fill in the blanks
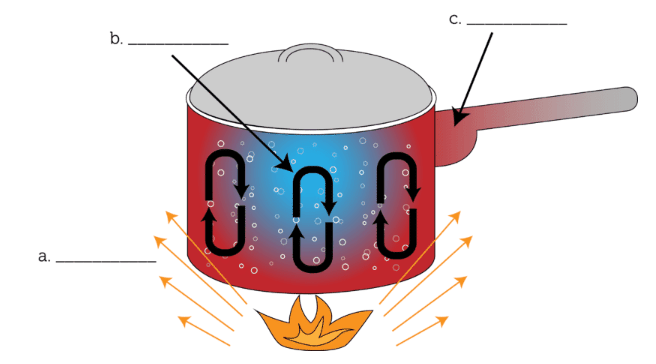
a) What is radiation
b) What is convection
c) what is conduction
In the designing a better cooler activity: The temperature after 4 hours is what variable? DV or IV
What is dependent variable?
Heat moves in one direction. How does an air conditioner show that?
What is an air conditioner removes heat from hot inside air, lowering the energy and temperature of the inside air.
Transfer of thermal energy between materials by direct collision of particles.
What is Conduction
Container A has 20 molecules with an avg. Ke (temp) of 25 C. Container B has 40 molecules with an avg. Ke of 25 C.
Which has a higher thermal energy and WHY?
What is container B because thermal energy is defined as total Ke of all molecules, and B has more molecules than A.
Why do homes in warm climates often have white roofs?
Prevent radiation by reflecting light/heat off the house
System: 10 C + 9 C + 11 C
What is the Temperature?
what is 10 C?
Average KE 10+9+11=30
30/3= 10
Which has More thermal energy:
- A 100L pot of 100 C water
- A 50L pot of 100 C water
What is a 100L pot of boiling water (more mass (molecules)= more energy!)
A large system with a total Ke of 6 when combined with a system with a total Ke of 7 will have a thermal energy of___.
What is 13?
The scale that Absolute Zero is measured on (hint: named after a dude)
What is Kelvin
System: 10 C + 9 C + 11 C Temperature
Thermal energy
What is 30 Kj?
TE is TOTAL energy 10 + 9 + 11= 30
You add cold water to your hot chocolate to cool it-what happens to the temperature?
What is it comes to equilibrium
A.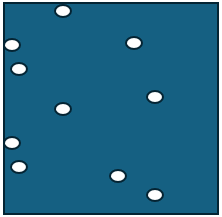
B.

does A have more thermal energy? Look closely....
What is NO. A has the same amount of molecules
Explain why the statement that ice cools down a glass of water is incorrect.
What is the water gives its energy to the ice, thus removing energy from the water cooling it down. Ice cools water by absorbing heat from the warmer water, a process of heat transfer that occurs because the ice is at a lower temperature. This absorption of energy is needed to melt the ice (a phase change from solid to liquid) and continues as the ice melts. The water's molecules slow down as they lose energy, causing the water to become cooler.
An air conditioner cooling down a room shows a system coming into equilibrium. True/False.
What is false. Air conditioner can cool a room LOWER than the outside temperature.
Your air conditioner breaks. The air in your room becomes warmer. System is coming to equilibrium. (True/False)
What is true? System is coming into equilibrium with outside temperature.