Vocabulary definition: A measure of the average kinetic energy of the particles of an object is its this. What is the vocabulary word?
What is temperature?
A system that allows matter and energy to transfer into surrounding areas
What is open system?
The process by which heat moves from a hand to an ice cube by direct contact.
What is conduction?
States of matter can change if _______ is added or taken away
What is heat energy
An object that conducts heat very well is classified as this.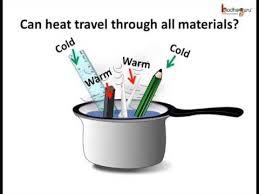
What is conductor?
Vocab Definition: the energy transfer from one object to another because of a temperature difference. What is the vocabulary word?
What is heat?
This thermal system traps in matter but allows heat energy to transfer into the outside surroundings
What is closed system?
A heater inside a hot air balloon heats the air and so the air moves upward. This causes the balloon to rise because the hot air gets trapped inside. When the pilot want to descend, he releases some of the hot air and cool air takes it place, causing the balloon to lower. Which form of heat transfer does the hot air balloon utilize?
What is convection?
If thermal energy is added to a solid, describe how the molecules will change.
Molecules will move faster (increase kinetic energy) and spread apart. The state of matter will turn to liquid or gas. More entropy.
Which state of matter has the greatest entropy?
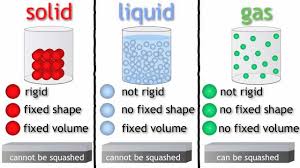
What is gas? (More disorder: more spread out and moving fastest)
Which law states that thermal energy flows from something at a higher temperature to something at a lower temperature
What is the 2nd Law of Thermodynamics?
A system that contains all matter and energy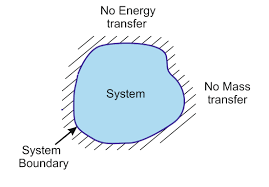
What is isolated system?
This form of heat transfer can travel through the vacuum of outer space through waves.
What is Radiation?
If heat energy is taken away from a gas, describe the molecular movement.
Molecules will move slower (decrease kinetic energy) and move closed together. The state of matter will turn to liquid or solid. Less entropy.
Name the three units of heat measurements
Celcius, Fahrenheit, Kelvin
This special, specific temperature has a lowest amount of kinetic energy.
What is absolute zero?
A green house allows sunlight in, but keeps its doors closed to prevent rodents. A greenhouse is a example of what type of system?

What is closed system?
Method of heat transfer when a person uses a hot compress.
What is conduction?
True or False - When thermal energy is added to molecules, the molecules will increase in size.
False- the size of the molecules will stay the same. The molecules will move faster and spread apart.
She has a headache and puts a cold spoon on her forehead. What will happen to the temperature of her hot head and the cold spoon?
The heat in from her head will move into the cold spoon. They will become the same temperature. (Thermal Equilibrium)
If these two beakers are both at 100 degrees Celsius, which would have the least amount of thermal energy?

Left Beaker!
The beaker with 300ml of liquid. This beaker has less molecules, therefore has less kinetic energy and thermal energy.
Energy can be transformed from one form to another within the system, but never exiting the system.
What is isolated system?
 In this picture of a convection current, which section of the boiling water is more dense with molecules that have the least amount of kinetic energy?
In this picture of a convection current, which section of the boiling water is more dense with molecules that have the least amount of kinetic energy?
The cooler water at the top of the kettle. (Blue arrow)
Thermal energy is the SUM of all molecular movement. Which would have more thermal energy; a lake or an ocean?
What is the ocean? Ocean has more molecules.
The cold spoon is pressed against her warm head. What is the difference in kinetic energy within the molecules on the spoon compared to the molecules of her head?
The molecules in the spoon are moving slower and are closer together. Molecules will have less entropy.
