This equation is used to calculate heat.
q=mCΔT
This is the definition of calorimetry.
What is the process of measuring the amount of energy released or absorbed by a substance?
This is the phase represented by region C.
What is a solid?
This is the definition of temperature.
What is the average kinetic energy of a sample?
This is 13,791 J in kJ .
What is 13.791 kJ ?
This is the standard unit for measuring heat.
What is a joule (J)?
These are the units specific heat is measured in.
What is J/(g*°C ) or J/(g*°K) ?
These are the phase changes represented at F, I, and K.
What is melting, condensation, and sublimation?
This is the difference in measurement of something in degrees Kelvin from degrees Celsius.
What is 273 degrees less?
This is the specific heat 43,110 kJ / (kg*K), converted to J / (g*C).
What is 43,110 J / (g*C) ?
These are the symbols in the equation:
q = mcΔT.
What is: q = heat, m = mass, C = specific heat, and ΔT = change in temperature ?
A block of hot metal is dropped into water at 25.0 degrees Celsius. Over the next minute, a student makes these observations of the water's temperature:
25.0
27.8
29.3
29.5
29.4
28.6
This is the temperature the metal and water reached thermal equilibrium, based on the data.
What is 29.5 degrees?
This is the phase of water at 370 degrees Celsius and 20 atmospheres. 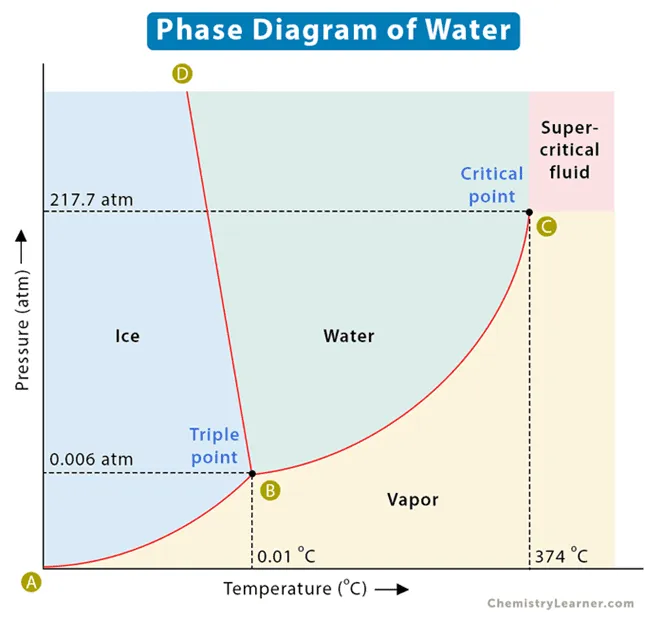
What is a gas/vapor?
This is the definition of heat.
What is the amount of thermal energy transferred?
This much heat is required to raise the temperature of 250.0g of mercury by 52.0°C (the specific heat for mercury is 0.14 J/g°C) .
What is 1,800 J ?
This is the heat when 30.0g of dry ice is cooled from -78.5 degrees Celsius to -125.0 degrees Celsius (the specific heat of dry ice is 0.815 J/(g*C).
What is -1,140 J ?
This would be the temperature change of 125.0g of steam, if you added 400.0J of heat to it (the specific heat of steam is 1.9 J/(g*°C) ).
What is 1.68°C ?
This is the spot on the phase diagram where a sample of matter can simultaneously have parts in solid, liquid, and gas phases.
What is the critical point?
This is the amount of energy it takes to increase one gram of a substance by one degree.
What is specific heat capacity?
This is the specific heat of a metal which has a mass of 25.0g and underwent a temperature change of 50.0 degrees Celsius when it absorbed 162.4J of heat.
What is 0.130 J/(g*°C)?
This is how much energy (in kJ) it would take to increase the temperature of 72.0 g of liquid water by 20.0°C (the specific heat of liquid water is 4.18 J/g°C).
What is 6.02kJ ?
A 150. g block of copper is heated to 95.0°C and then placed into 220 g of water at 25.0°C. The water and copper reach thermal equilibrium at 30.0°C. Assuming no heat is lost to the surroundings, this is the specific heat capacity of copper.
What is 0.47 J/(g*C) ?
This is the conditions of CO2 when it's in a supercritical phase.

What is a temperature over 31 degrees Celsius AND a pressure over 73.8 bars?
This is the ability to do work.
What is energy?
This is the final temperature of 56.6g of iron when 4,892 J of energy is absorbed from it at 31 degrees Celsius (the specific heat of iron is 0.45 J/(g*C) ).