Defined as the energy of motion.
Kinetic Energy (KE)
In the formula, ▵U = Q - W, what does the "▵U" indicate?
Change in total internal energy of the system.
"Heat always flows from a hot object to a _____ _____."
Cold or colder object.
A hot coffee cup left by mistake on a kitchen counter for an hour. Which is the system? Which are the surroundings?
Coffee cup is the system; the kitchen is the surroundings.
You burn your hand when you grab a metal spoon left in a pot of chicken soup cooking on the stove. What type of heat transfer is this?
Conduction.
Defined as energy that is stored and held ready to go.
Potential energy (PE)
In the formula, ▵U = Q - W, what does the "▵Q" indicate?
Heat added to or heat lost by the system.
What does "irreversible" mean with a process?
A process that cannot be completely reversed and returned to its initial state.
A pot of chicken soup cooking on the stove. What type of system is this?
Open system.
Roasting marshmallows over a campfire to make s'mores with your friends. What type of heat transfer is this?
Radiation.
This energy depends on mass and height.
Gravitational potential energy (GPE)
What two (2) variables drive the total internal energy change of a system?
Adding heat to the system or doing work to the system.
Defined as a measure of a system's disorder or dispersion.
Entropy
A tightly sealed thermos with hot chocolate contained inside. What type of system is this?
Isolated system.
Ocean currents bring warmer waters from the Gulf Stream to the Atlantic Ocean. What type of heat transfer is this?
Convection.
In the image below, what energy transfer is being depicted?:
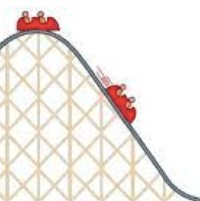
Gravitational Potential Energy (GPE) to Kinetic Energy (KE)
In the specific heat formula, Q = s x m x △ T, what does the "Q" indicate?
The heat energy required by a substance.
When heat is transferred from a hotter object to a colder object, we say that the ________ increases.
Entropy
A crock pot cooking your favorite beef stew. What type of system is this?
Closed system.
Defined as the measure of the average kinetic energy of individual particles of matter.
Temperature.
The unit of measure for Kinetic Energy (KE).
Joules
(or 1 kilogram x meters2/seconds2)
In the formula, ▵U = Q - W, when is the "W" value negative? (Using the convention we have established)
When the surroundings do work on the system.
What does "spontaneous" mean in Physics?
A naturally occurring process that happens without outside energy input.
When the system exchanges energy but not matter with its surroundings, this is an example of a(n) ________ system.
Closed system.
Defined as the movement of thermal energy from a substance at a higher temperature to another at a lower temperature.
Heat.