Term defining point 1, also indicated by (λ = lambda)
What is wavelength
The orbital that holds 2 electrons
What is the s-orbital
 the letter that represents the number of protons and the number of electrons
the letter that represents the number of protons and the number of electrons
What is Z?
A subatomic particle that has 0.0005 relative mass and a relative charge of -1
What is an electron?
Atoms of the same element with differing amounts of neurons
What is an isotope
A characteristic pattern of spectral lines given when atoms of a single element are provided with energy.
What is emission spectra
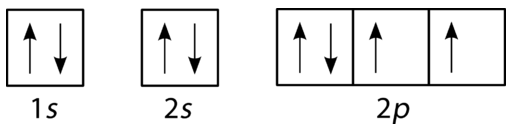 The orbital diagram of this element
The orbital diagram of this element
What is the orbital diagram of oxygen?
This is defined as the number of protons in the nucleus.
What is the atomic number?
These become farther apart when nearing the nucleus in a hydrogen atom.
What are energy levels for an electron in a hydrogen atom?
The number of electrons present in 40Ca2+
What is 18 electrons?
Number of wave cycles per second that pass a given point (ν = nu)
• Unit is s-1 (per second)
What is frequency
The full electron configuration of this element is 1s2 2s2 2p6 3s2 3p6 4s2 3d2
What is Titanium
The amount of valence electrons are present in an atom of an element with atomic number 16
What are 6 valence electrons?
In the hydrogen atom, this what happens to the energy levels as they increase in energy.
What is convergence?
the number of protons in a fluoride ion19F-
What are 9 protons?
The relative atomic mass of rubidium. 
What is 85.46
The element in Group 17 and period 5 and has the configuration [Kr]5s24d105p5
What is the electron configuration of Iodine
This is the number of neutrons, given that the mass number of an aluminum atom is 27 and the atomic number is 13.
What are 14 neutrons
In the hydrogen atom, this is the energy needed to remove an electron from the ground state of each atom in a mole of gaseous atoms, ions, or molecules.
What is ionization energy?
There are this many neutrons in the ion 18O2-
What are 10 neutrons
These represent the energy (or wavelengths/frequencies of visible light) emitted by an electron as it transitions from a higher to lower energy level.
What are the colored lines on an emission line spectrum?
The ground-state electron configuration of the Fe3+ ion.
What is 1s22s22p63s23p63d5
The number of each of the sub-atomic particles present in atom of 226Ra.
What are 88 protons, 88 electrons, and 138 neutrons?
After the excited state is produced, this is the state/level in which an unstable electron falls back to.
What is the ground state (lowest level)?
The relative atomic mass of magnesium containing 79% 24Mg, 10% 25Mg, and 11% 26Mg.
What is Mr = 24.32