What classification of matter can be broken down into simpler substances only through a chemical reaction?
Compound
Name a separation technique used to separate a liquid from an undissolved solid, like sand from water.
Filtration
Chemical bonds are a form of intermolecular forces, True or False?
False - they are intramolecular forces
Categorize the following as an Element, Compound, or Mixture: Brass
Mixture
This separation technique relies on the differing solubilities and attraction to a stationary phase, often used to separate colors in ink
Chromatography
What property of a substance is a direct measure of how easily a liquid evaporates into a gas?
Vapor Pressure or Volatility
This term describes a mixture where the composition is uniform throughout, meaning you cannot see the different components.
Homogenous mixture
You have a homogeneous mixture of salt dissolved in water. Besides distillation, what method would you use to recover only the salt?
Evaporation
You are performing the penny water drop experiment: there are 2 trials, one with pure water and one with a mixture of soap and water.
Which penny will hold more droplets and why? 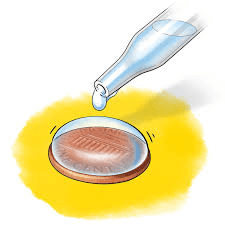
The one with pure water because the soap interrupts the intermolecular forces of water, lowering the attraction between water molecules.
Categorize the following as an Element, Compound, or Mixture: Cl2 gas
Diatomic element
Why is the process of Distillation able to successfully separate a mixture of two liquids?
Because of their different boiling points
(Honors Only) Which specific type of dipole-dipole force occurs when a hydrogen atom is bonded to a highly electronegative atom like Nitrogen (N), Oxygen (O), or Fluorine (F)?
Hydrogen Bonding
What would a particle level model for a Mixture: Two Elements look like?
A model showing two different types of unbonded atoms, with all particles mixed together
If you are separating a mixture of two liquids using distillation, the component that is collected first is called what?
Distillate
(Honors Only) Acetone is a polar molecule with dipole-dipole forces, and Propane is a nonpolar molecule with only dispersion forces. Which liquid would have the lowest vapor pressure and why?
Acetone, because stronger IMFs (dipole-dipole) hold the molecules together more tightly, making it harder for them to evaporate and therefore resulting in the lowest vapor pressure