The smallest unit of matter.
Atom
The subatomic particle with a positive charge
proton
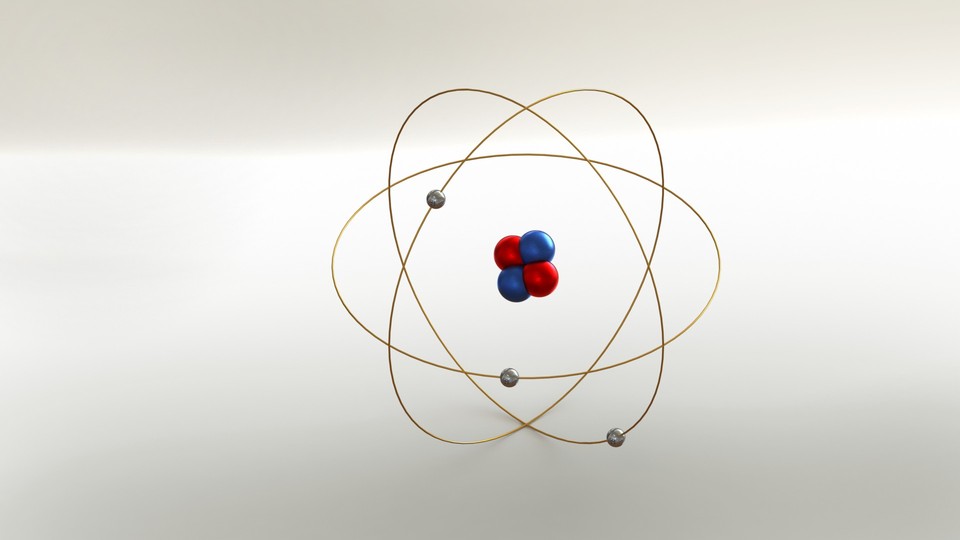
How many protons are in the atom, below?
3 protons
Isotopes of a particular element have different numbers of
neutrons
Atomic number is the number of
Protons
subatomic particles
The subatomic particle with a negative charge
electron
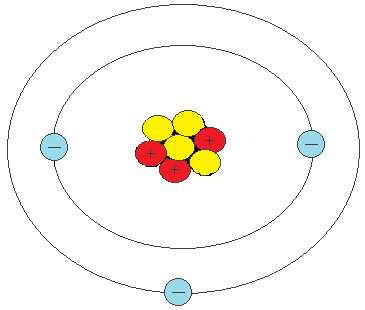
How many electrons can be found in this atom?
3 electrons
Isotopes of the same element always have the same number of
protons
Mass number is the number of
protons + neutrons
What can be found outside of the nucleus in a cloud?
Electrons
The nucleus of an atom has a _______ charge.
positive
How many neutrons can be found in Carbon-14?

8 neutrons
Are the two elements below, Isotopes or different elements?
Element C - 8 p+ and 9 n0
Element D - 10 p+ and 9 n0
Different elements
What is the mass number of this atom?
4
mass number = p+ + n0
The center of an atom is called
nucleus
What two subatomic particles are attracted to each other?
protons (+) and electrons (-)
How many neutrons can be found in this isotope of oxygen?
*HINT: We have not learned about 2-, yet.
8 neutrons
16 - 8 = 8
Are the two elements below, Isotopes or different elements?
Element E - mass number is 3 and atomic number is 4
Element F - mass number is 3 and atomic number is 3
Different Elements
What is the atomic number of neon?
10
What two subatomic particles contribute to most of the mass of an atom?
Protons and Neutrons
The charge of an atom with equal protons and electrons.
neutral
How many neutrons does silicon-29 have?
![]()
29 - 14 = 15 neutrons
What does the number after the element, below, represent?
Carbon - 12
Mass Number
How many neutrons does Aluminum have?

14 neutrons
27 - 13 = 14
27 (rounded atomic mass)
14 (atomic number = # of protons)