What does it mean when pH = pKa?
Exactly half the acid has dissociated. Buffering region is +/- 1.
What does it mean to be oxidized or reduced?
OIL RIG
Oxidized: Loss/Donor of electrons
Reduction: Gain/Acceptor of electrons
Give examples of two catabolic and two anabolic metabolic pathways.
Catabolic: Generate ATP & reducing power
-glycolysis, PPP, fatty acid oxidation
Anabolic: Require ATP & reducing power
-gluconeogenesis, fatty acid synthesis, cholesterol synthesis
Name 2 positively charged and 2 negatively charged amino acids.
(+) Lysine, Arginine, Histidine
(-) Aspartate, Glutamate
In a redox reaction, how will you know which half-reaction will proceed as a reduction?
Higher standard E value
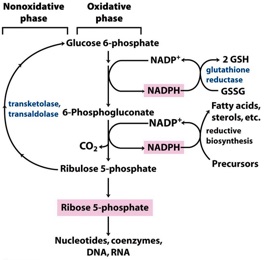
Purpose of the Non-Oxidative phase of PPP?
Non-oxidative converts 5-C sugars back to 6-C sugars (for more NADPH synthesis) or generates glycolysis intermediates (for ATP synthesis)
What is the isoelectric point of lysine? pKa's: 2.18, 8.98, 10.53
9.74
List three things ∆G tells you about a reaction.
a. How far the reaction is from equilibrium
b. How much energy will be released as the reaction proceeds toward equilibrium
c. In which direction a net reaction will occur (if it does occur)
i. Negative ∆G – reactants to products (exergonic)
ii. Positive ∆G – products to reactants (endergonic)
*Does NOT tell you about reaction rate*
Purpose of Oxidative phase? Under what conditions would it be increased?
Oxidative phase generates NADPH (biosynthesis and prevent oxidative damage^) and pentose-P (RNA & DNA)
 *Increased in tumors to provide nucleotides for increased cell division
*Increased in tumors to provide nucleotides for increased cell division
*Increased in Red Blood Cells which have high concentrations of oxygen
What types of inhibitors affect Vmax and Km?
Vmax: Uncompetitive and Mixed
Km:All! Competitive, Uncompetitive, and Mixed
.jpg) Competitive: Only binds to E
Competitive: Only binds to E
UnCompetitive: Only binds to ES
Mixed: Binds to both
Describe the 2 anaerobic fates of pyruvate (include enzymes and products)
a. Lactate dehydrogenase: pyruvate + NADH + H+ --> lactate + NAD+
b. Pyruvate decarboxylase (+TPP)/alcohol DH: pyruvate + NADH --> ethanol + CO2 + NAD+
What is the purpose of the ETC? Summarize how this is accomplished in three steps.
Purpose is to convert reducing power to ATP!
i. Electrons from NADH and FADH2 are shuttled to O2
ii. Producing H2O and resulting in a H+ gradient
iii. H+ gradient drives ATP synthase
Explain how insulin and glucagon regulate blood glucose levels (including pathways stimulated by each hormone).
Insulin – decreases blood glucose (glycolysis, glycogen synthesis, cholesterol synthesis, fatty acid synthesis – leading to TAG and PL synthesis)
Glucagon – increases blood glucose (gluconeogenesis, glycogen breakdown)
List ways that anabolic pathways are regulated within the cell
a. Allosteric regulation: end product of pathway inhibits enzyme catalyzing rate-limiting step
b. Reversible covalent modification: for example, phosphorylation/de-phosphorylation can activate/inactivate enzymes that catalyze committed (rate-limiting) steps in pathway
c. Gene expression: Insulin signaling activates some genes involved in biosynthesis and inactivates genes involved in glucagon signaling
Explain two ways hemoglobin is allosterically regulated.
1) Binding of oxygen creates a conformational change to stabilize the R-state.
2) Binding of 2,3-BPG creates a conformational change to stabilize the T-State
Describe what DNP does to O2 consumption and ATP synthesis
Uncouples electron transport and ATP production => increased O2 consumption and little ATP synthesis
i. Dissipates proton gradient => ATP production is reduced
ii. Cell increases catabolic reactions to try to reach ATP needs
iii. Increased body heat
What is the net reaction of the light reactions? What is the purpose of each component going into the light reactions?

NET: light energy + H2O + NADP+ => NADPH + ATP + O2
b. Light energy – produces high energy electrons
c. H2O – donates electrons => proton pumping (ATP) and reducing power (NADPH)
d. NADP+ accepts electrons
Fructose-2,6-bisphosphate increases the activity of which enzyme and stimulates which pathway?
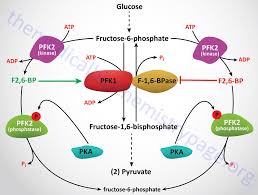
-Increases activity of PFK-1
-Stimulates the conversion of F6P => F16BP (Glycolysis)
-On the opposite side F26BP decreases FBPase-1 activity, inhibiting conversion of F16BP à F6P (i.e. Gluconeogenesis)
Explain how chylomicrons, LDL, and HDL differ in composition and function.
a. Chylomicrons: largest, lowest density, mostly TAGs, carry dietary fats to tissues
b. LDL: medium size, low density, most cholesteryl esters, delivers cholesterol to muscle, adipose tissue, and can result in formation of foam cells if it gets stuck in arterial walls.
c. HDL: smallest size, highest density, mostly phospholipids and proteins, picks up cholesterol and returns it to the liver. Can help reduce plaque size.