What is an independent variable?
The Variable that is changing in the experiment.
Which 2 Macromolecules gives living things energy?
Lipids and Carbohydrates
Water has a much higher specific heat than most other molecules. What do you predict might happen if water had a low specific heat instead?
A.Flooding would occur and animals would be forced to migrate.
B.Harmful organisms living in water would reproduce at a rapid rate.
C.Organisms that are sensitive to changes in temperature would die.
D.Plants would not have enough water to effectively carry out photosynthesis.
C.
An enzyme is a _______ Which means it speeds up reactions
Catalyst
There are 5 students outside, three are wearing a white shirt and 2 students are wearing a black shirt. I can see that the two in a black shirt are sweating. What can I infer about this observation?
Black shirts are hotter than white shirts
What is the dependent variable in an experiment
The outcome, what is being measured
Which Macromolecule is this?
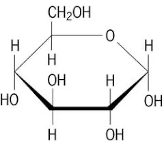
Carbohydrate
What property of water allows plants to bring water up through its roots?
Capillary Action, Cohesion and Adhesion
Which reaction used an enzyme? Redline or blackline 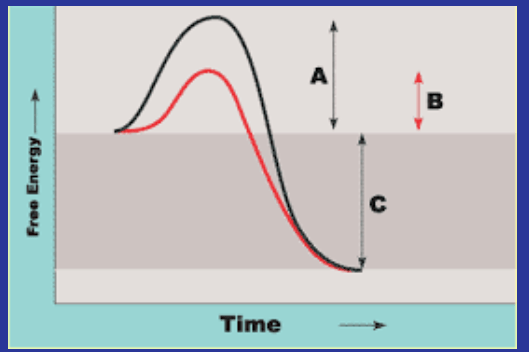
Red line
Enzymes are what Macromolecule?
Protein
What is a control group and why is it important
It is the group that has nothing done or what is our normal, it is out baseline to see if the independent is actually doing anything.
Which Macromolecule is this?
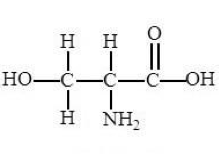
Protein
What property of water allows nutrients to travel through the body
Universal Solvent
What is the Optimum pH for this enzyme?
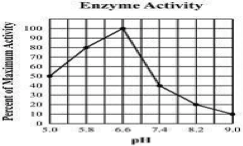
6.6
What Macromolecule is this?
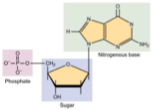
Nucleic Acid
What is an observation based off this image

There is rain drops on the leaf
What is the monomer for this Macromolecule?
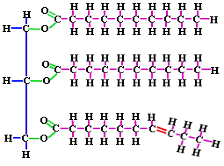
Fatty Acid
Many fish and aquatic plants can survive a cold winter because the layer of ice that forms at the top of the lake insulates the water below and prevents the lake from freezing solid. What unique property of water contributes to this effect?
A.Water absorbs heat when it evaporates and forms a gas.
B.Water expands and becomes less dense when it freezes.
C.Water molecules break apart into ions in solutions.
D.Water forms hydrogen bonds with ions and other polar substances.
B.
Why are enzymes essential for life?
It allows the body to be able to do all the processes that are required inside our body.
What property of water allows water to be considered a universal solvent?
Polarity
What is an inference based on this image?

It rained, someone watered the plant
Which of the following macromolecules is NOT matched correctly with its function?
A.Carbohydrates = short term energy, component of cell wall
B.Lipids = long term energy, component of cell membrane
C.Proteins = provide energy needed for muscle growth and protein synthesis
D.Nucleic acids = stores genetic information and involved in protein synthesis
C
Why does a belly flop into a pool hurt?
A. Adhesion causes water molecules stick to your belly very quickly.
B. The specific heat of water burns your skin.
C. Water dissolves other substances which float to the top creating a barrier.
D. Cohesion of water molecules forms surface tension on the water.
D.
What happens when an enzyme exceeds their optimum temperature or pH?
It denatures
What property of water allows living things to moderate their temperture?
High specific heat