What is the volume of the toy in mL using proper precision?
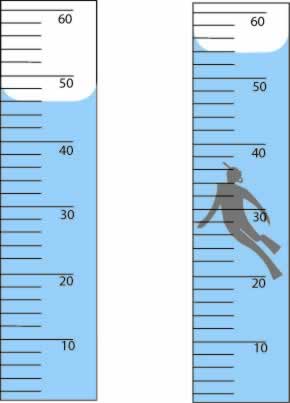
What is 8.0 mL
What is the equation for density?
What is D= m/v
What type of heat transfer is the example of heat being given off by a lightbulb?
What is radiation
What is the phase change occurring at B as the substance goes from A to C?

What is melting
Find the heat absorbed by 3.10 g of water that increases in temperature from 27.0 oC to 95.0 oC. The specific heat of water is 4.184 J/g oC.
What is 882 J
The temperature shown below (to proper precision)
What is 32.0°C?
What happens to density if mass increases, but volume stays the same?
What is increases
What type of heat transfer is the example of your feet walking on asphalt on a hot day?
What is Conduction
What is the phase change occurring at D as the substance goes from E to C?

What is condensation
Find the heat absorbed by 16.90 g of water as it goes from 95.0 oC to 47.0 oC. The specific heat of water is 4.184 J/g oC.
What is -3,390 J
You want to measure the volume of a paper clip. The water level in a graduated cylinder starts at 7.95 mL, then rises to 8.4 mL. What is the volume of the paper clip in mL using correct sig figs?
What is 0.5 mL
What happens to density if volume decreases, but mass stays the same?
What is increases
What type of heat transfer causes tectonic plates to move?
What is convection
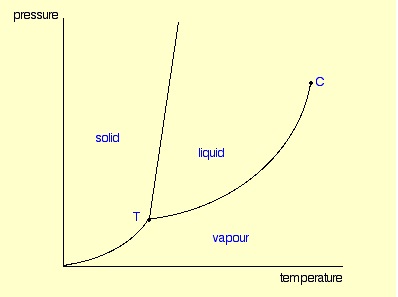 The x-coordinate of a point on the line between the solid and liquid areas represents the __________ of the substance at a certain pressure.
The x-coordinate of a point on the line between the solid and liquid areas represents the __________ of the substance at a certain pressure.
What is the melting/freezing point?
What is the final temperature of 12.0 g of water that started at 3.50 oC and absorbed 459 J of energy? The specific heat capacity of water is 4.184 J/g oC.
What is 12.64 oC
What is the volume of a rectangular box with the measurement 11.00 cm, 4.80 cm, and 4.55 cm? Report your answer in proper units and sig figs.
What is 240. cm3
Calculate the density of an object that has a mass of 19.24 g and a volume of 7.93 mL? Make sure to include units and put your answer in significant figures
What is 2.43 g/mL
What type of chemical process (endothermic or exothermic) is the example of fire burning?
What is exothermic
When a substance is going through a phase change, what is happening on a molecular level?
A 2.50 g sample of zinc is heated, then placed in a calorimeter with water. The water absorbs 685. J of energy, and reached a final temperature of 22.50°C. If the specific heat capacity of zinc is 0.390 J/g°C, what was the initial temperature of the zinc sample?
What is 725°C
Round the following number to 3 significant figures: 569600
What is 5.70 x 105
A chef wants to know the volume of oil they need for a dish. The recipe states that 25.0 g of oil is needed. If the density of oil is 0.92 g/mL, how many mL of oil does the chef need?
What is 27 mL
What type of chemical process (endothermic or exothermic) takes place in an instant ice pack?
What is endothermic
When a substance is increasing in temperature, what is changing at a molecular level?
What is a change in speed (kinetic energy)
A 15.0 g block of metal starts at 105.0 oC and is dropped into 50.0 g of cold water. The temperature of the water started at 30.8oC, then increased to 34.0 oC after the metal was added. The specific heat capacity of water is 4.184 J/goC. What is the specific heat of the metal? Include units!
What is 0.63 J/g oC