Elements with similar properties are typically found
a) same group
b) same period
c) opposite sides of PT
d) diagonal on PT
a) same group
For any atom, how many electrons can occupy the 3d orbital?
10
What is the electron configuration of a Fluorine atom? (write the full configuration, NOT noble gas)
1s22s22p5
What is the ENC experienced by a 2p electron in an F- anion?
7
What is the trend for atomic radius as you move from bottom left to top right of the periodic table?
Atomic radius decreases
What does quantum number "ℓ" tell us?
orbital shape
Which of the following represents Aufbau Principle?
a) no 2 electrons can have the same quantum numbers (must have opposite spin)
b) electrons in degenerate orbitals will put 1 electron in each orbital before pairing up
c) electrons fill the lowest energy levels first
c) electrons fill the lowest energy levels first
What is the electron configuration of a Selenium atom? (Noble gas)
[Ar] 4s23d104p4
What is the ENC of a valence electron in the Al+ cation?
3
Rank the following isoelectronic series in order from LARGEST to SMALLEST ionic radii:
Na+, N-3, Mg+2, O-2, F-1
N-3, O-2, F-, Na+, Be+2
What are the boundary conditions for mℓ?
+ to - of ℓ value
-3, -2, -1, 0, 1, 2, 3
Which of the following represents Pauli Exclusion Principle?
a) no 2 electrons can have the same quantum numbers (must have opposite spin)
b) electrons in degenerate orbitals will put 1 electron in each orbital before pairing up
c) electrons fill the lowest energy levels first
a) no 2 electrons can have the same quantum numbers (must have opposite spin)
What ion of Sulfur would have the electron config below?
1s22s22p63s23p6
S-2
How many shielding electrons are there in a Mg atom?
10
Rank the following from LEAST to GREATEST ionization energy: Si, P, S
Si < S < P
Allowed or not allowed quantum numbers?
n= 1, ℓ = 3, mℓ = 0, ms = -1/2
NOT allowed
ℓ = n -1
Which of the following represents Hund's Rule?
a) no 2 electrons can have the same quantum numbers (must have opposite spin)
b) electrons in degenerate orbitals will put 1 electron in each orbital before pairing up
c) electrons fill the lowest energy levels first
b) electrons in degenerate orbitals will put 1 electron in each orbital before pairing up
Which of the following atoms would be diamagnetic?
K, Al, Mg, Cl
Mg
How many shielding electrons are there in a Br- anion?
28
Order the following in order of INCREASING electronegativity:
C, P, S, F
P, S, C, F
How many electrons could be described by the quantum numbers n = 2, ms = +1/2
4
Correct or Incorrect?
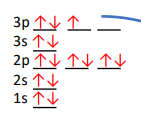
INCORRECT - violates Hund's rule
Which of the following would you predict to be the most magnetic?
Sc, Ti, V, Mn
Mn
Which of the following is NOT isoelectronic with the other ions?
H-2, Be+1, Na+8, Li+1
Li+1
Which of the following elements is the MOST electronegative?
K, Cs, Li, Na
Li