1 liter is this many times larger than a milliliter.
1000
The elements in the modern periodic table are arranged in this format.
Increasing atomic number
This person was the first to describe the atom.
Democritus
The number 505020 has this many significant figures.
5
This is the unit used to measure mass in the metric system.
grams
4.56 g = 4560 mg
This is considered a(n) ______________________.
conversion factor
This group of elements is nonreactive.
noble gases/group 8
The electron was discovered by this person after his work with cathode ray tubes.
J.J Thomson
6.82 x 104 has ________ significant figures.
3
This person developed the first model of the periodic table.
Dmitri Mendeleev
A megameter is ______ times larger than a meter.
1 million
An atom of sulfur has this many valence electrons in its outer shell.
Six
This subatomic particle determines the identity of an atom.
proton
The number 0.0002500 will have a ___________ exponent when written in scientific notation.
negative
The particles in this state of matter have the most energy.
Gases
Conversion factors are useful for this reason
They help express the same value using different units.
Li (lithium) is ________ electronegative than F (fluorine).
less
This element has this atomic number

37
276000 written in scientific notation is
2.76 x 105
How many electrons are found in arsenic's (As) outer shell?
5
This is the system used in all scientific measurements.
The Metric System
This explains that atoms tend to form compounds in a way that gives them a full valence shell like that of noble gases.
The following isotope of krypton has this many neutrons.

42
The first rule of determining significant figures states this
all non-zero digits will ALWAYS be significant
The following is the Bohr model of the element ____________.
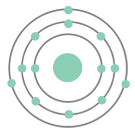
Aluminum