Uncertainty
Uncertainty
Analysis
The tool of primarily used in chemistry to measure liquids.
What is a graduated cylinder?
The amount of sig figs in the number: 0.089
What is 2?
For the exponential number in a scientific notation number to be positive, we had to move the decimal to the ______
What is left?
There are _______ cm in 6.8 in
What is 17.27 cm?
Density is a relationship between ____ and ____
What is "mass" and "volume"?
Multiplication and division calculations need to be reported to the least number of _______.
What is Sig Figs?
The number of certain numbers in the measurement of this graduated cylinder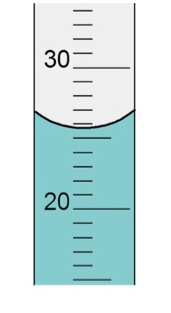
What is 2?
The amount of sig figs in the number 6.02
What is 3?
For the exponential number in a scientific notation number to be negative, we had to move the decimal to the ______
What is right?
There are _____ miles in 900 ft
What is 0.17 miles?
An irregular shaped object's volume can be measured using the ________ method
What is "liquid displacement"?
Addition and subtraction calculations need to be reported to the least number of _______.
What is decimal places?
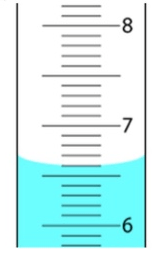
The uncertainty of the following picture is ________
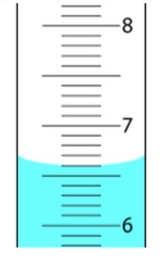
What is +- 0.01?
For a zero to count as a sig fig it must follow a _____ and there must be a _____ present in the number
What is "non-zero number" and "decimal"?
The number 5063 in scientific notation is ______
There are ______ muffins in 17.2 dozen muffins
What is 206.4 muffins?
If an object with a volume of 6.0 mL is dropped into the following graduated cylinder, what will the final measurement of the graduated cylinder be?
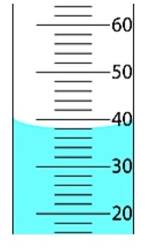
What is 44.0 mL?
The answer for the calculation 22.5 x 6.8 x 11.2 is _________.
What is 1700? (Or 1.7 x 103)
The amount of Sig Figs the following measurement would need to be reported with:
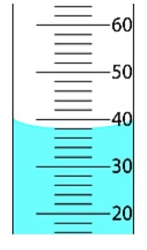
What is 3?
The answer for 3.56 / 7.822 is _______
What is 0.455?
The number 0.657 in scientific notation is ______
What is 6.57 x 10-1?
There are ______ seconds in 8 hours
What is 28,800 seconds?
An object with a volume of 2.50 mL was dropped into this graduated cylinder. What was the measurement of the graduated cylinder BEFORE dropping the object in it?
What is 4.10 mL?
The answer for the calculation 200.5 - 12.35 is _____.
What is 188.1?
When 2.5 mL are taken away from the first graduated cylinder the final measurement is ________
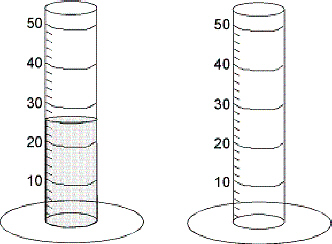
What is 23.5 mL?
The answer for 6.7 x 8.92 is ______
What is 6.0 x 101?
The number 5.6 x 10-3 in NORMAL notation is _______
What is 0.0056?
There are _____ seconds in 4.2 years
What is 132,451,200 seconds?
A 457 g sample with the following dimensions has a density of __________.

What is 0.457 g/mL?
The answer for the calculation (4.40 x 6.12) + (9.8 / 3.00) is ________.
What is 30.2?