What phase of matter is being shown here:

Solid
What are the two factors that influence the strength of the electrostatic force?
Distance and Charge
Which element has 13 protons?
Aluminum
What does this symbol mean?![]()
Partial Charge
Why do metals conduct electricity?
They have loosely held electrons that are able to move freely, allowing charge to flow.
What is the capital of Oregon?
Salem
Fill in the blank: Gas has high _______ meaning it has a large amount of disorder.
Entropy
What is the name of the equation for finding the strength of an electrostatic force?
Coulomb's Law
Find the missing information: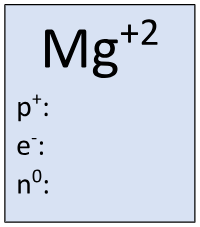
p = 12
e = 10
n = 12
How do you ionize something?
Add or remove an electron
What does Effective Nuclear Charge (ENC) mean?
The actual pull on the outer electrons from the nucleus.
What state is this? 
What happens to the energy of particles as a liquid turns into a gas?
It increases
If the distance between two charged particles is cut in half, what happens to the strength of the force between them?
It increases by 4
Draw a bohr model for Nitrogen.
Image to be drawn up front.
When someone is rubbing a balloon and their sweater, what is happening at the atomic level?
Electrons are being transferred because of frictional forces.
What do the electrons of insulators do when an electric current is applied?
They vibrate and generate heat.
Who sings this song? (Bonus 100 points if you can name both)
Dua Lipa and Elton John
Free electrons & Positive Ions
Why do metals have a less firm hold on electrons compared to non-metals?
Complete the missing information: 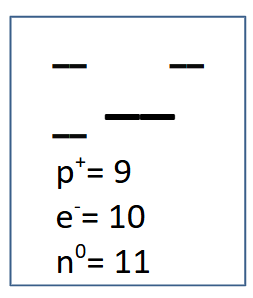
Fluorine, atomic mass 20, atomic number 9, charge -1
Name 2 examples of polarized objects we have discussed.
A wall, the ground, water molecules, soda can, etc.
Draw how this image would change if an electrical current was applied:
What is the population of Bellingham? (within 3 thousand is correct)
94,720 measured in 2023 at least...
What is the term for when a solid turns directly into a gas?
Sublimation
When electrons are pushed close together, where does the kinetic energy of movement go?
Into the electric field between the electrons.
Write the nuclear symbol for two different isotopes of the Boron
What causes water to be polar?
The oxygen atom holds the electrons closer to the nucleus.
Why does adding salt to water allow it to conduct electricity?
It adds ions (charges) to the liquid that move freely when a current is applied.
What movie is this image from?![]()
The Truman Show