What is the smallest building block of all matter?
An atom
What does a subscript tell you?
How many atoms of each element there are
Which kind of change results is a change in physical properties?
Physical change
What is an aqueous solution?
A solution where the solvent is water
What is the difference between an element and a compound?
Elements only have one type of atom, while compounds have more than two different atoms
How many atoms are in this formula?
H2O
Three atoms:
Hydrogen = 2
Oxygen = 1
Name at least 3 evidence of a chemical change
When making lemonade, lemon juice is the _______.
Solute
Cl2 is a _______. (TWO ANSWERS)
Molecule and element
How many SULFUR (S) atoms are in this formula: H2SO4
1 atom
Which kind of change is very difficult or impossible to reverse?
A fully mixed hot cup of tea would be a _________.
Solution or Aqueous solution
What kind of particle is this? TWO ANSWERS.
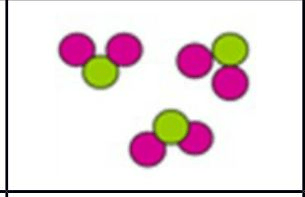
Molecule and compound
How many atoms are in this formula: C4H10
14 atoms
Chemical change
Concentration
How many compounds are in this formula?
CH4 + Cl2 --> CCl4 + HCl
Three compounds because Cl2 is a molecule.
How many atoms are in this formula: 2CaH2PO4
16 atoms
Ca=2, H=4, P=2, O=8
When two liquids mix and create a solid, that is called a ___________.
Precipitate
If I wanted to dilute my cup of coffee, I would add more __________