These is the units used to describe volume of liquids.
What is milliliter (mL)?
This technique separates solid and liquid through a filter
What is filtration?
This type of matter can not be broken down any further.
What is an element?
This type of change involves a change in appearance but no change in its chemical make up
What is a physical change?
The number of significant figures in the measurement 245 g.
What is 3?
This instrument is used to measure mass.
What is a scale?
What is distillation?
This type of mixture is also described as a solution, since you can not see the different components.
What is a homogenous mixture?
This type of property can only be observed through a reaction.
What is a chemical property?
This is the direction that you move the decimal when an exponent is negative.
What is left?
This is the equation for used to determine a substances' denisty.
What is mass/volume?
This technique uses heat to evaporate a liquid and leave behind a solid.
What is watch glass/crucible evaporation?
Carbon dioxide and sulfur hexafluoride are classified as these types of matter.
This is an example of this type of change.
What is a chemical change?
This is the number of significant figures in the number 101,030.0
What is 7 significant figures?
The method of finding the volume of an irregular object, like a rock, by placing it in a graduated cylinder with water and measuring the change in the water level.
What is water displacement?
What method would be best used to separate two colorless liquids?
What is distillation?
Greek salad would be classified as this type of matter
What is a heterogenous mixture?
This property of wood describes its ability to burn and turn into ash and smoke.
What is flammability?
This is the correct format for 0.0000675 in scientific notation.
What is 6.75 x 10-5?
This is the name of this type of glassware.

What is an Erlenmeyer flask?
This technique separates particles by size and solubility.
What is chromotography?
This image represents this type of substance.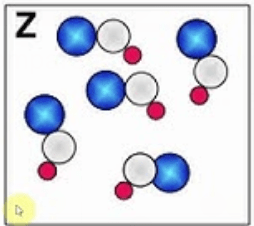
What is a pure compound?
This type of physical change involves a solid turning to a gas without a liquid phase.
What is sublimation?
The number 6,020,000,000 written in proper scientific notation.
What is 6.02×109?