This particle is located in the nucleus of an atom and has a mass of 1 amu, and a charge of +1.
What is a proton?
What charge is a proton?
Positive
distinguish between a nuclear reaction and a chemical reaction
Chemical reaction normally occurs outside the nucleus. Nuclear reaction happens only inside the nucleus. or ____________
Define half life
the amount of time it takes HALF of a substance to decay into a set of more stable atoms.
What is an isotope?
Atoms of the SAME element but with different masses
Atoms contain __________ subatomic particles
a) 1
b) 2
c) 3
3
What charge is a electron?
Negative
What are the three types of radiation that can be emitted during a nuclear reaction?
alpha
beta
gamma
What must be true when writing a balanced nuclear equation?
mass and atomic numbers are conserved
Calcium
This particle is found flying around the nucleus in orbitals.
What is an electron?
What charge is an Neutron
Neutral
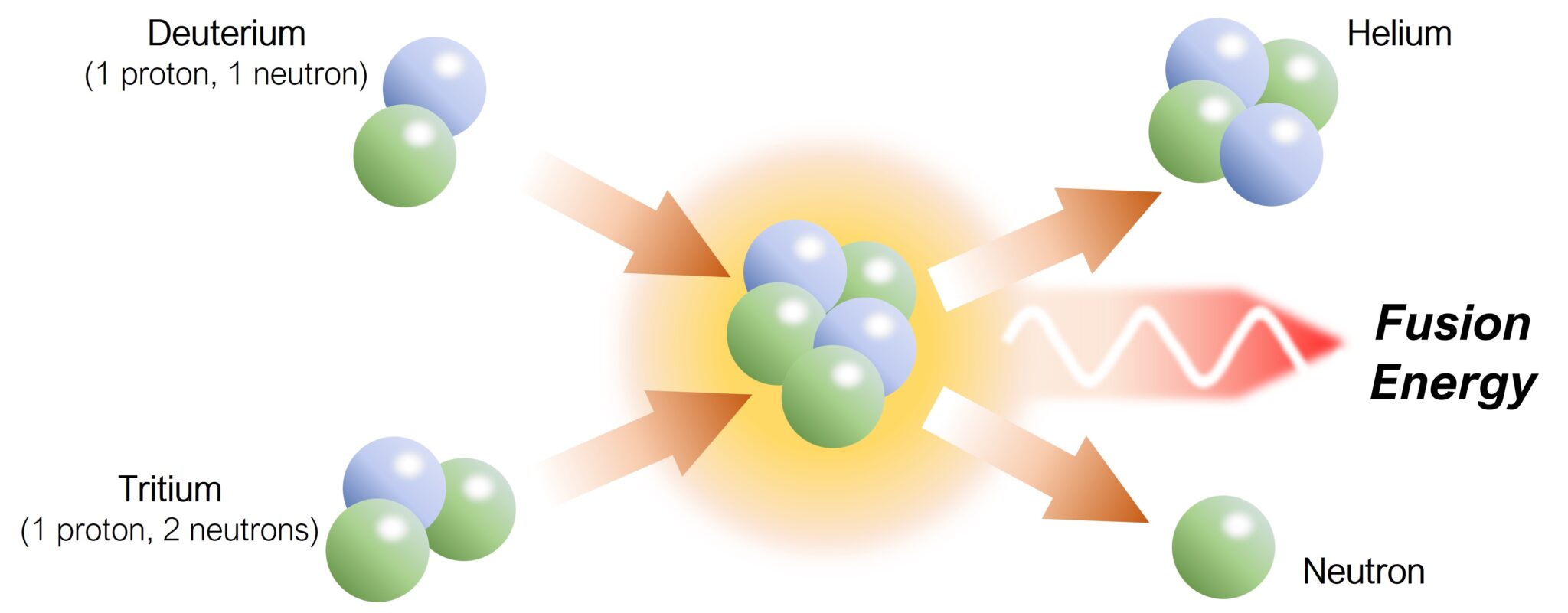 identify the type of reaction shown above.
identify the type of reaction shown above.
fusion
Determine the missing particle:
22288Ra --> _____ + 42He
21886Rn
![]()
Nitrogen
These particles have NO charge but have a mass of 1 amu.
neutron
Does an electron in an atom move at all?
Yes-Electrons move quickly
Determine the type of radioactive decay occuring below
Alpha decay
Determine the missing particle
189O --> 189F + ____
Which particle determines the identity of an element?
protons
Which particle does NOT change the identity of an element, only its mass?
neutron
What are the three basic particles of an atom?
Protons, electrons, and neutrons
Why do atoms undergo radioactive decay?
large ratio of neutrons to protons
to gain stability
15 days
The atom is held together by _____ –
mainly the electromagnetic force and the
strong nuclear force.
Forces