What is the density of water?
1.0 g/cm3
Name a conversion factor for mass.
1 lb = 453.6 g
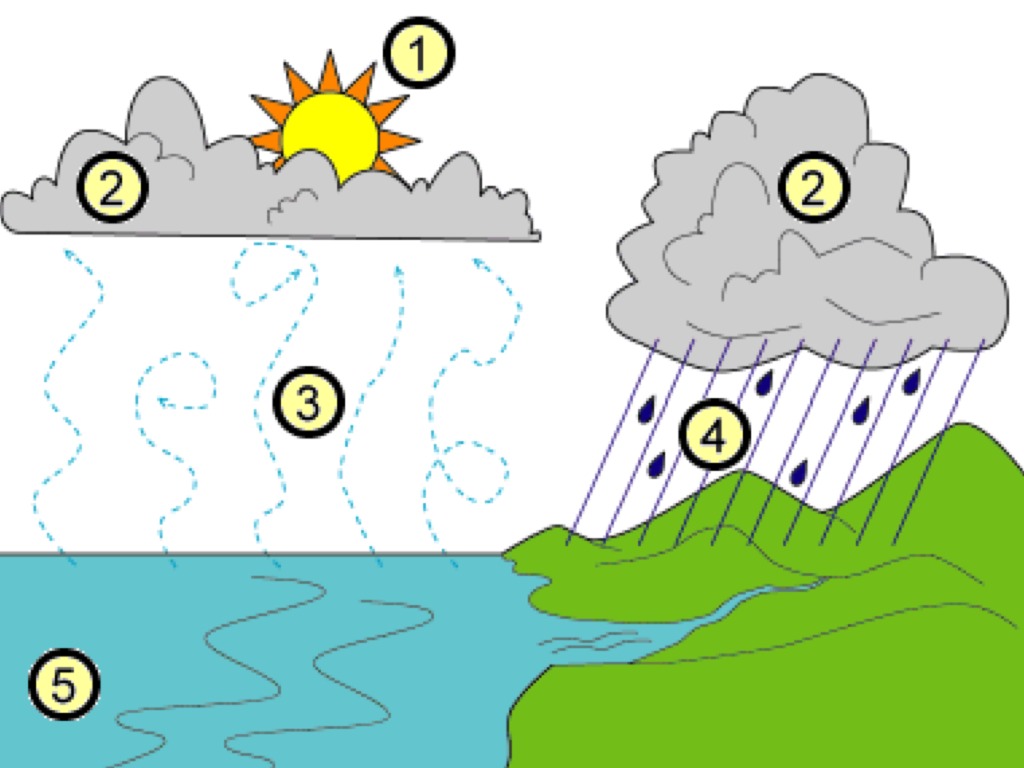
What is happening in stage 2?
Condensation
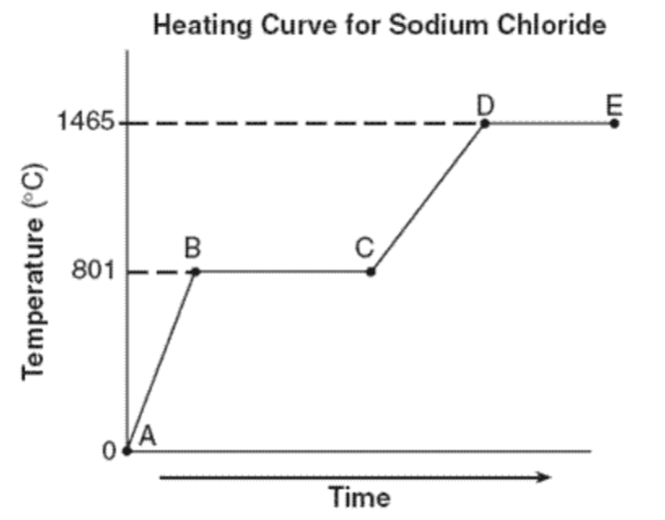
What is the freezing point (temperature) of sodium chloride?
801 0C
Where is most of Earth's fresh water located?
Glaciers
Calculate the density of a substance that has a mass of 3.5 g and a volume of 15 mL.
0.23 g/mL
Convert 100 mg to g.
You must show me your fence method on a piece of paper to earn the points.
0.1 g
What is the evaporation off the leaves of plants called?
Transpiration
What state(s) of matter are present during D-E?
liquid and gas
Name two indirect uses of water.
Using kleenex, using electricity
Calculate the volume of a 500 g nugget of gold that has a density of 19.3 g/mL.
25.9 mL
Convert 45 s to days.
You must show me your fence method on a piece of paper to earn your points.
0.00052 days
Describe one way that humans can decrease the rate of percolation.
Increase the amount of pavement, cut down trees
What is happening to the kinetic energy during stage A-B?
increasing
Name two places in Michigan that have polluted water. Give the cause of the water pollution as well.
1. Flint - Lead in water (past)
2. Ann Arbor - dioxane plume in aquafer by Jackson Road
3. Grand Rapids Area - Wolverine Boot Factory - PFAS contamination in ground water
What does increasing the temperature do to the density of an object?
The density decreases
Convert 34 g to oz.
You must show your fence method on a piece of paper to receive credit.
1.20 oz
Name two ways to decrease the amount of runoff.
1. plant vegetation
2. decrease the slope
What is happening to the distance between the particles of sodium chloride during segment B-C?
Increasing. The particles are moving farther apart.

What is the volume of the hammer?
69 mL - 65 mL = 4 mL
Calculate the mass of a cube of aluminum with a density of 2.7 g/mL that measures 4.3 cm on each side.
214.7 g
Convert 30 km/hr to m/s.
You must show your fence method on a piece of paper to earn the points.
8.3 m/s
Give two effects increased global temperature would have on the water cycle.
1. increased rate of evaporation
2. increase in transpiration
3. increase in precipitation due to more water vapor in the atmosphere
Why is segment D-E longer than segment B-C?
It takes more energy to boil a substance than melt it because upon boiling ALL intermolecular bonds must be broken vs. during melting only a few have to be broken.
What is the volume in the cylinder?
6.32 mL