Which rheological layer of the earth exhibits plasticity and undergoes heat transfer via convection?
the asthenosphere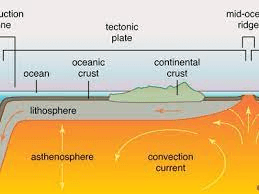
What is the type of thermal energy movement that involves the circulation of fluids?
Convection
The process of a liquid becoming a gas is known as
Evaporation (or vaporization)
Define temperature
The measure of the average kinetic energy of an object's particles.
Provide an example of radiation.
Getting sunburnt, holding your hands near a fire, sitting in a tanning bed...
Complete the definition:
A mineral is _____________.
a naturally occurring crystalline solid.
What happens during a subduction?
This is when two plates with different densities collide. For instance, when the oceanic plate collides with the continental plate, the oceanic plate will go under (subduct) the continental plate as it is more dense.
What happens to the temperature of the substance as it undergoes a phase change?

The temperature stays constant during a phase change.
How would you compare the kinetic energy of particles between a gas and a solid?
The energy in a gas' particles are higher than that of a solid.
This graph shows reactants absorbing energy. What type of reaction is this?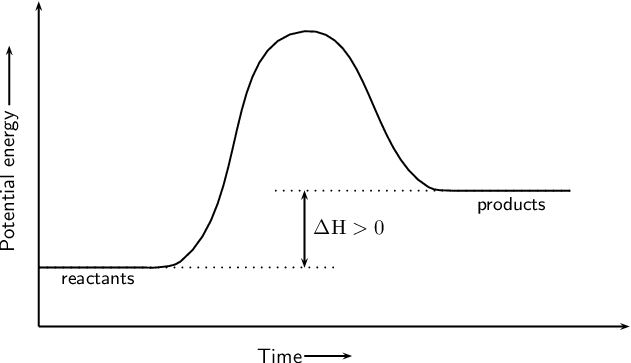
The heat is being absorbed in the system. Therefore, this is considered as an endothermic reaction.
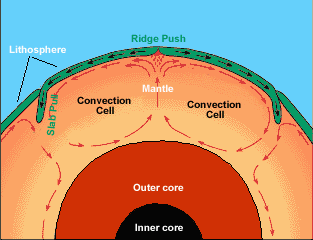
The picture above illustrates that _______ is the primary motivator of plate motion.
Convection
This is the Thingvellir fracture zone in Iceland, which is considered a rift. What type of plate boundary do you think this will exist? How do you know?

Divergent Plate Boundaries, a rift forms, when two plates are moving away from each other.
Calculate the heat required to melt 205 g of Ice.
Given that Water's ΔHvap: 2257 J/g
Water's ΔHfus: 333.6 J/g
Values can be negative depending on the phase change
Useful Equations
q = mΔvaporization
q = mΔfusion
68,400 J
quantity of heat needed to change the temperature of 1 g of a substance by 1 degree Celsius
specific heat (c)
Based on your knowledge of rocks, what is the name of this rock?
granite (or granitoid)
What type of plate boundary exists between the North American Plate and the Eurasian Plate?
a divergent boundary.
Yes, this is due to the fact that there is high silica and felsic composition which will lead to a more viscous magma. A more viscous magma will promote higher pressure which will increase the chance of volcanic eruptions.
How much energy being absorbed in Joules is required to heat 50.0 grams of iron with a temperature change of 55°C? The specific heat of iron is 0.444 J/g°C.
1220 J
When 2 different temperature objects are placed in close proximity to each other, energy flows from the ______ object to the ______ one.
energy moves from the warmer object to the cooler one.
Calculate the Standard Heat of Formation (ΔH) for this reaction. Is this exothermic or endothermic?
SO3 (g) + H2O (g) --> H2SO4 (aq)
Given that the ΔH of:
H2SO4: -909.27 kJ
H2O: -241.8 kJ
SO3: -395.7 kJ
Answer: -271.77 kJ (Exothermic)
Work:
H2SO4 ---> H2O + SO3
(-909.27) - (-241.8 + -395.7) = -271.77 kJ
Along certain parts of the Ring of Fire, the Pacific Plate is converging with the North American Plate and the Australian Plate. What type of geological landform would you expect to see here?
trench / subduction zone
Convection occurs in which of earth's layers? (there's more than one)
Outer core and mantle (asthenosphere)
How much energy is released when 25.0 grams of aluminum metal is cooled from 125°C to 35°C? The specific heat of aluminum is 0.900 J/g°C.
Useful Equations
q = mc(Tfinal - Tinitial)
Answer is -2030 J
When you place a piece of ice on a piece of plastic and a piece of aluminum that are both the same temperature, you notice that the ice melts faster on the aluminum. What property of the aluminum is responsible for this phenomenon?
Aluminum's thermal conductivity is higher than plastic's!
What kind of curve is the illustration below? Where is the direction of heat in this diagram? Why do you think so?
This is a cooling curve and the direction of heat is leaving the system. As a result the ΔH values for each phase change is negative.