Which state of matter has a definite volume, but no definite shape?
A liquid
Which phase change process occurs when a substance changes from a liquid to a gas?
Boiling/Vaporization
If an object has a density of 3 g/ml, will it sink or float in water?
Sink
As the volume of a container of gas goes up, what will happen to its pressure?
It will decrease.
What is the first step of the scientific method?
Ask a question
Which state of matter is a gas that consists of electrically charged particles?
Plasma
In an exothermic process energy is __________________.
(absorbed or realeased)
realeased
If an object has a mass of 60g and a volume of 22 ml, what is its density?
2.73 g/ml
As the temperature of a cars tire decreases, what will happen to its pressure?
it will decrease
True or false: You should NOT make a volume measurement with a beaker.
True
Between solid, liquid and gas, which has the LEAST amount of energy?
solid
What is an example of a phase change process that is endothermic?
Melting or Boiling
If an object has a mass of 30 g and a volume of 32 ml, will it sink or float in water?
Float
A balloon is tied to a rock and dropped into the ocean. What will happen to the volume of the balloon?
Hint: As you get deeper into the ocean, the pressure increases
The volume will decrease
Which type of variable changes as a result of the manipulation of another variable?
Dependent
Between solid, liquid and gas, which has the MOST amount of energy?
Gas
Which phase change process occures when a solid changes directly to a gas?
Sublimation
If an object has a density of 0.875 g/ml and mass of 21 g, what is the objects volume?
24 ml
Descibe the relationship between volume and pressure.
inverse relationship, as one goes up, the other goes down
What is the SI unit for time?
seconds (s)
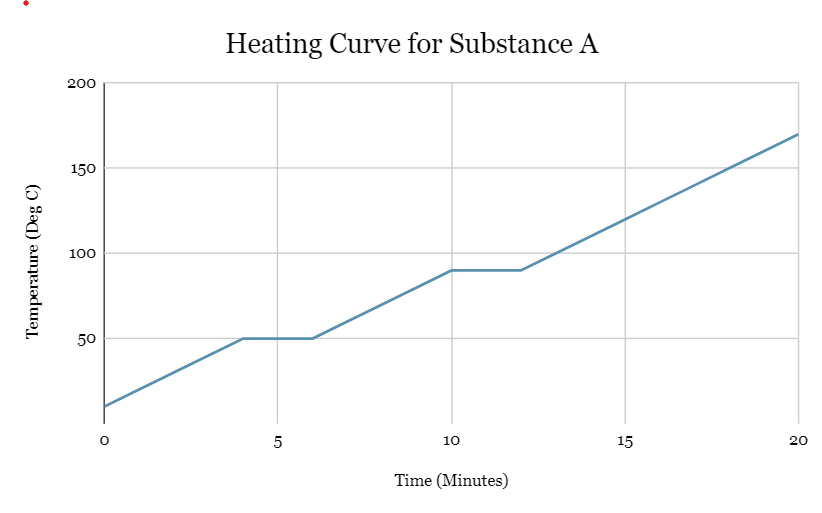
In what state is substance A at 150oC?
Gas
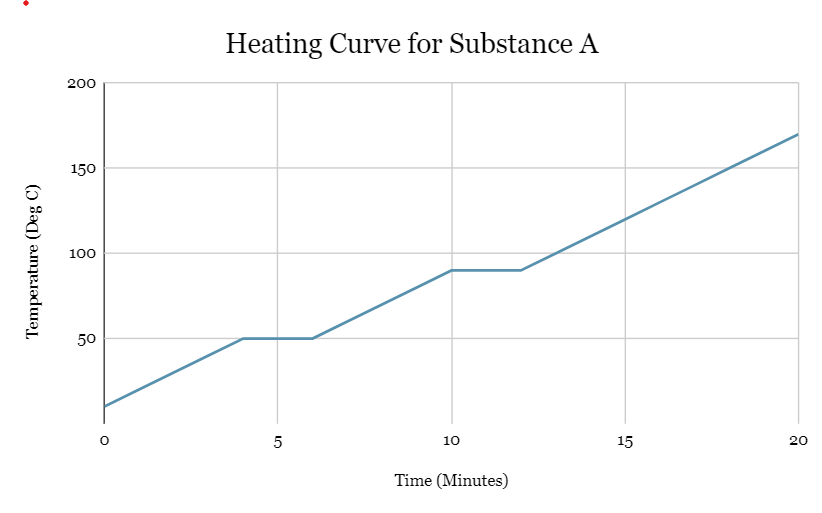
What is the melting point of substance A?
50oC
Before a rock is placed in a graduated cylinder, the volume of water in the cylinder is at 32.3 ml. After the rock is placed in the graduated cylander, the volume is 35.6 ml. If the mass of the rock is 17.16 g, what is the rock's density?
5.2 g/ml
In a hot air balloon, fuel is ignited, causing the air around it to heat up. The hot air is then directed into a large balloon, and the balloon begins to rise. Why does the balloon rise in terms of gas laws?
Hint: Think about the density of the gas
Name a piece of lab equipment that you would use to make a length measurement.
Ruler, Meter stick, measuring tape, etc