When writing scientific notation, what is my base number?
x 10 n
Boiling point, melting point, and density are all examples of ____________.
intensive properties
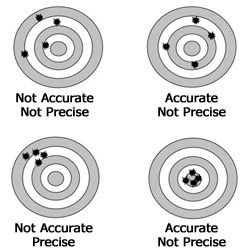
Write down the definition of accuracy.
Accuracy is how close a measurement is to the true or accepted value.
What are the 3 states of matter?
Solid, liquid, and gas
What school did Ms. Molina teach at last year?
Elsik
Why do we write numbers in scientific notation?
All chemical properties are going to be __________ (intensive or extensive).
intensive
Write the definition of precision.
Precision is how close the measurements are to one another.
What are the two classifications of matter?
Pure Substance and Mixtures
What sport does Ms. Molina coach?
Tennis
Calculate the number of sig figs in the following number:
0.0201
3 sig figs
List 3 types of chemical properties.
flammability, reactivity, toxicity, sensitivity to heat/light, etc.
Ms. Molina was trying to do an experiment to calculate the density of aluminum (2.7 g/mL). Based on her results, was she accurate, precise, both, or neither?
2.924, 2.923, 2.925, 2.926
precise - values are closer to 3 g/mL instead of 2.7 g/mL
Where can I find my elements? Give 3 examples of an element.
Periodic table; answers will vary.
What college did Ms. Molina attend?
University of St. Thomas
Change the following number from standard notation to scientific notation:
45,400
4.54 x 10 4
List the following 3 pictures as physical or chemical changes.

physical, chemical, chemical

Which of the following pictures shows low accuracy but high precision?
B
True or false, if false, please correct the statement: Homogeneous mixtures allow you to see the individual parts and heterogeneous mixtures are the same throughout.
False, homogeneous mixtures are uniform throughout and heterogeneous mixtures are different throughout.
What country is Ms. Molina's family from?
Bolivia
Write the following number in standard form AND list the number of sig figs.
6.77 x 10-2
0.0677
3 sig figs
Give an example of a physical property, physical change, chemical property and chemical change.
Physical property: color, mass, volume, length, density, MP, BP, etc.
Chemical property: reactivity, flammability, toxicity, acidity, sensitive to light/heat, etc.
Physical change: paper ripping, wood being chopped, viscosity, ice cream melting
Chemical change: burning wood, baking a cake, milk turning sour, fireworks, digestion, etc.
Draw a picture of a target that is accurate but NOT precise. Show at least 5 targets.
helium, olive oil, air, vitamin C
element, mixture, mixture, compound
What is Ms. Molina's favorite sports team?
Astros