What are 2 ways to find the Atomic Number of an atom?
Locate the number of Protons and/or Electrons.
What element is shown below?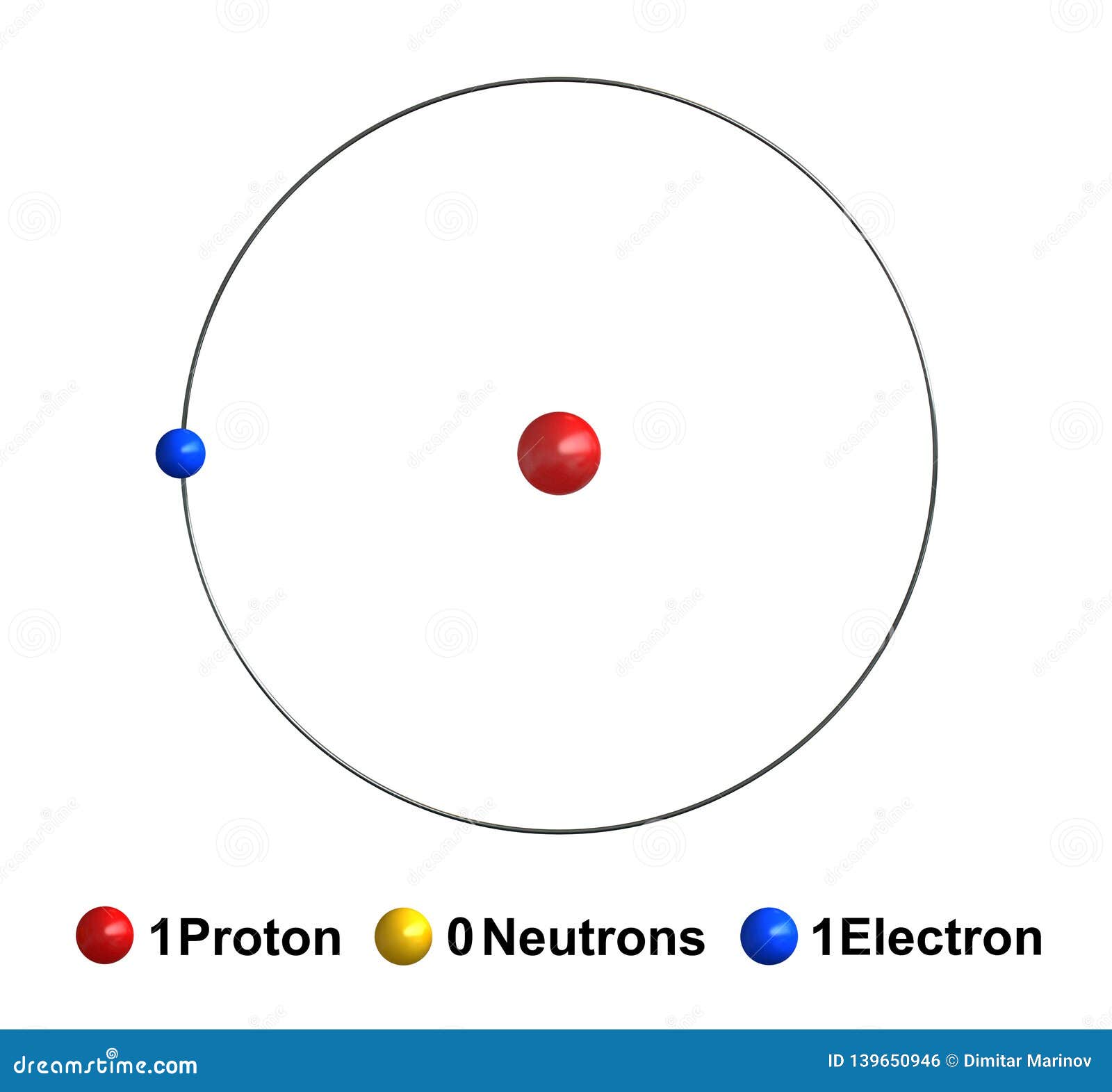
Hydrogen
How many electrons can you have on each energy level of an atom?
Level 1: 2
Level 2: 8
Level 3: 8
What element has 2 energy levels and 3 valence electrons?
Carbon (C)
What is the name of the group of elements on the periodic table that are non-reactive?
Noble Gases
What is the atomic number and name of an element with 10 electrons and 10 protons?
10, Neon
What element is shown below?

Silicon
What is the name of the subatomic particles found in the last ring of an element?
Valence electrons
What is a group and how many are located on the periodic table?
Vertical columns on the periodic table indicated by the number at the top of the chart. Every element has the same Valence electrons. There are 18 total groups.
What do you call the 3 main sections of the periodic table?
Metals, non-metals, metaloids
If an atom has 39.95 mass and an atomic number of 18, how many neutrons does that element have?
22n0
What would a Bohr model of Sodium (Na) look like?
APE= 11, Mass= 22.99(23), N= 12

How can you determine how many energy levels an element has?
Find what period it is on and/or calculate the number of electrons in the atom.
What is a period and how many are located on the periodic table?
Horizontal rows on the periodic table indicated by the numbers on the left or right of the chart. Each period has the same energy levels. There are 7 periods total.
How do you determine the reactivity of an element?
Elements on the periodic table become more reactive as you move out from the center (groups 3-12).
If you do not know what the mass of an element is, but you do have the atomic number and neutrons, how can you determine the atomic number?
Atomic number + neutrons = Mass
What 2 subatomic particles are located in the nucleus of an atom?
Protons and Neutrons
If an element has 3 energy levels and 5 Valence electrons, how many total electrons are in that element? What element would that be?
15, Phosphorous (P)
Which 2 elements are on the same group and which 2 are on the same period?
Lithium, Oxygen, Nitrogen, Sulfur
Group: Lithium, Nitrogen
Period: Oxygen, Sulfur
List the groups on the periodic table from most to least reactive (not including 3-12).
1, 17
2, 16
13, 15
14
What does APE MAN stand for and how does it help you to locate elements?
APE: atomic number = protons = electrons
Man: mass - atomic number = neutrons
Its used to find the atomic number and mass to identify its location.
What are the parts that make up an atomic model?
Nucleus: protons and neutrons
Energy levels: electrons
What period is this element located on?
Period 4 because of its 4 energy levels
What are 2 elements that have the same Group and Period number?
Hydrogen and Berylium
Where are metaloids located on the periodic table?
The staircase: Boron, Silicon, Phosphorous, Arsenic, Selenium, Tellurium, Iodine, Astatine.