Picket Fence
The number of significant figures:
2,987
What is 4?
Convert the number to scientific notation:
0.00199
What is 1.99 x 10-3
Determine the mass in grams:
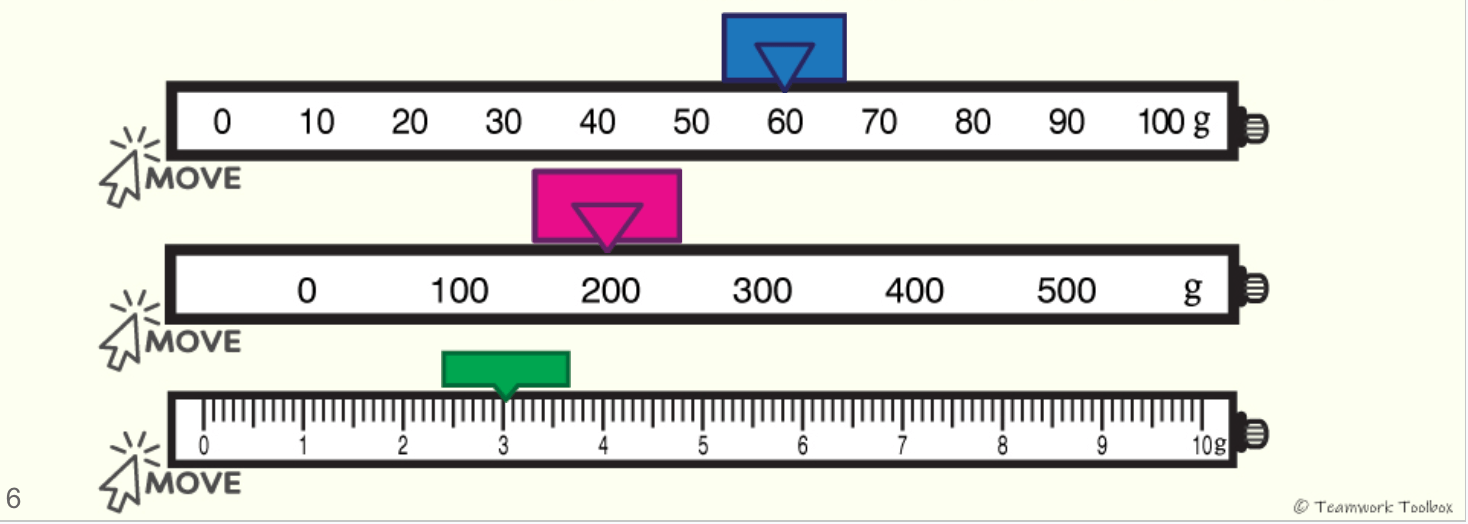
What is 263.3 grams?
Convert 4 mm to meters. Show your picket fence.
What is 0.004 m?
Determine the classification of orange juice with pulp.
What is a heterogenous mixture?
You cut a piece of paper into small pieces.
Determine if this is a physical or chemical change.
What is a physical change?
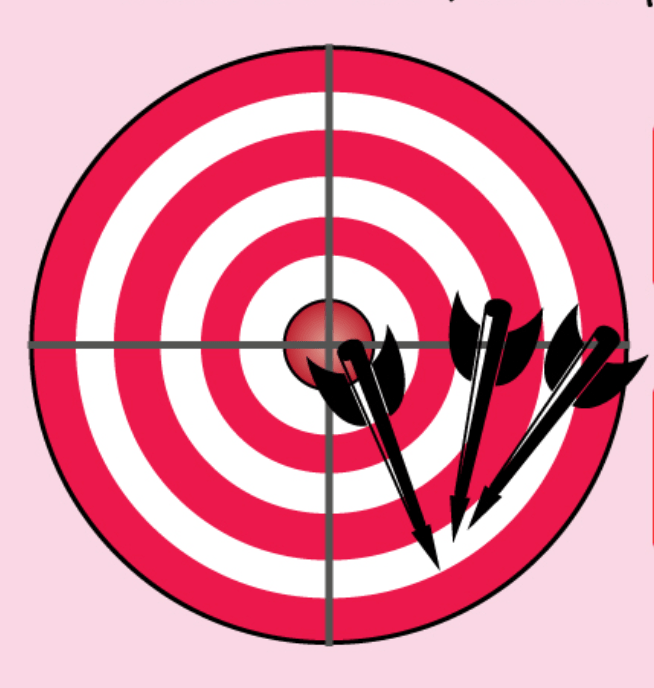
Describe the accuracy and precision.
What is high precision and low accuracy?
The number of significant figures:
58,000
What is 2?
Convert the number to standard notation:
2.998 x 108
What is 299,800,000?
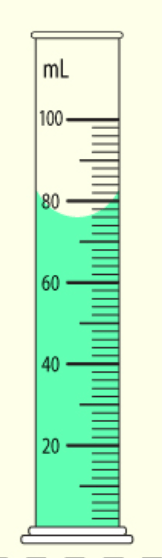
The number of millimeters in this graduated cylinder.
What is 76.0 ml?
Convert 1600 L to kiloliters. Show your picket fence.
What is 1.6 kL?
Determine the classification of gold.
What is an element or pure subbstance?
You find a nail on the ground in the parking lot, in a small puddle of water. Rust has formed on the outside of the nail.
Determine if this is a physical or chemical change.
What is a chemical change?
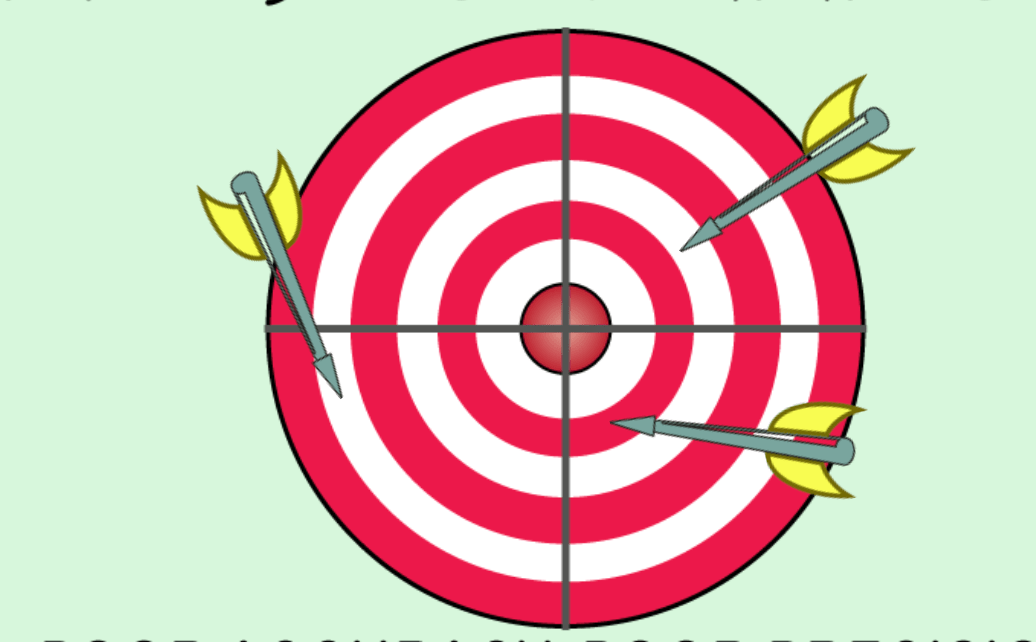
Describe the accuracy anf precision.
What is low accuracy and low precision?
The number of significant figures:
10,465
What is 5?
Convert the number to standard notation:
7.12 x 10-2
What is 0.0712?
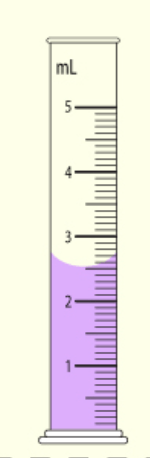
The number of millimeters in this graduated cylinder.
What is 2.51 ml?
Convert 2050 cm to kilometers. Show your picket fence.
What is 0.0205 km?
Determine the classification of sodium chloride (NaCl).
What is a compound or pure substance?
You pick up an ice cream sandwich from the school cafeteria and stick it in your hoodie pocket so your hands are free to hand the cash over to the cafeteria worker. You run into your chem lab partner on your way to your seat, and he tells you your homework is due next period. You forget all about your ice cream until you pull the plastic full of mush out of your pocket later that afternoon.
Determine if this is a physical or chemical change.
What is a physical change?
Jack, a snack food manufacturer, produces bags of potato chips, each measuring 11 oz. He tests the weight of the bags using a scale. There is a slight variation in the measurements: 12.2 oz, 12.33 oz, and 12.13 oz for three samples. Describe the accuracy and precision of Jack's measurements.
What is low accuracy, high precision?
The number of significant figures:
0.00873
What is 3?
Convert to scientific notation?
0.00880000
What is 8.80000 x 10-3?
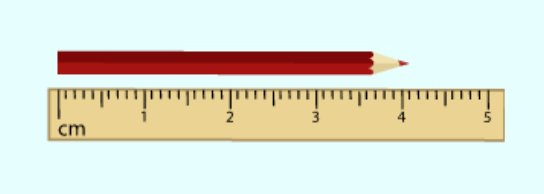
The length of the pencil in centimeters.
What is 4.10 cm?
Convert 0.986 hours to seconds. Show your picket fence.
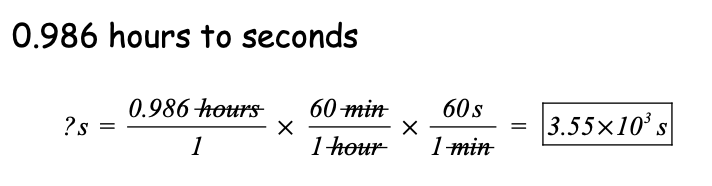
Determine the classification of bronze. Bronze is an alloy of copper and tin.
What is a homogenous mixture?
Determine the following property as extensive or intensive:
Density
What is an intensive property?
The following measurements were made to determine the density of a material whose value was, according to the Handbook of Chemistry and Physics, 1.15 g/mL
Trial #1 0.95 g/mL
Trial #2 1.16 g/mL
Trial #3 1.26 g/mL
Describe the accuracy and precision of these measurements.
What is low accuracy and low precision?
The number of significant figures:
102,430
What is 5?
Convert to standard notation:
5.15 x 10-5
What is 0.0000515?
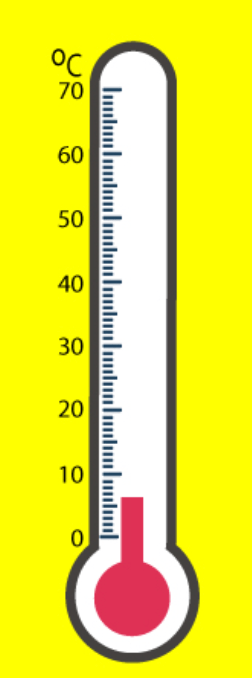
The temperature reading of the thermometer in degrees Celsius.
What is 6.5 degrees Celsius?
Convert 56,000 micrograms to kilograms. Show your picket fence.
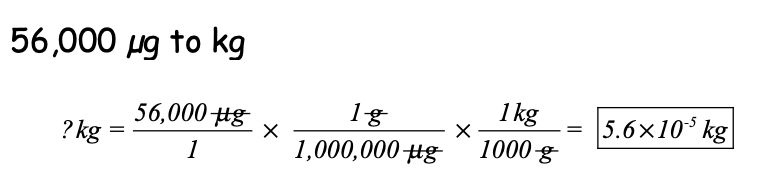
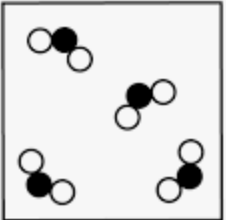
Determine the classification of the particle diagram.
What is a compound, or pure substance?
Determine the following property as extensive or intensive:
Volume
What is an extensive property?
A student took a calibrated 200.0 gram mass, weighed it on a laboratory balance, and found it read 196.5 g. Determine the student’s percent error.
What is 1.75%?