What is the sate of matter of substance B?
Gas
 Put the three objects in order from MOST dense to LEAST dense.
Put the three objects in order from MOST dense to LEAST dense.
CBA
What is the formula for Density?
D = M/V
What is the atomic number of Bismuth? 
83
______ refers to the way a metal can be hammered or rolled into thin sheets.
Melleable
 Identify substance A and describe its Kinetic Energy.
Identify substance A and describe its Kinetic Energy.
Substance A is a liquid, it has more kinetic energy than a solid but less than a gas.
Medium kinetic energy.
Given the following densities, rank the liquids from highest to lowest density:
- Orange Juice: 1.03 g/cm³
- Milk: 1.03 g/cm³
- Vinegar: 1.01 g/cm³
- Water: 1.00 g/cm³
Ranked from highest to lowest density:
- Orange Juice: 1.03 g/cm³
- Milk: 1.03 g/cm³ (same density as Orange Juice)
- Vinegar: 1.01 g/cm³
- Water: 1.00 g/cm³
M = 21 g V = 2 cm³ Density = ______
10.5 g/cm3
 What CLASS is outlined in blue
What CLASS is outlined in blue
Metals
Nonmetals are...
A) Shiny, Malleable, Conductors
B) Dull, Brittle, Insulator
C) Shiny or Dull, Brittle, Conductors
D) Dull, Malleable, Conductors
Rank the kinetic energy of Solids, Liquids, and Gasses. The one with greatest kinetic energy being first.
Gas, Liquid, Solid
You have two objects: one is a rock with a density of 2.50 g/cm³, and the other is a piece of cork with a density of 0.25 g/cm³. If you put both objects into a container of water, which object will float and which will sink?
What is the density of water?
The rock with a density of 2.50 g/cm³ will sink in water because it is denser than water. The piece of cork with a density of 0.25 g/cm³ will float because it is less dense than water.
The density of water is 1.00 g/cm³
M = 44 g V = 4 cm³ Density = ______
11 g/cm3
 Look at the provided picture. What type of element do the green elements represent?
Look at the provided picture. What type of element do the green elements represent?
Metalloids
Metals are...
A) Shiny, Malleable, Conductors
B) Dull, Brittle, Insulators
C) Shiny or Dull, Brittle, Semiconductor
D) Shiny, Dull, Insulator
Explain the shape and volume of a gas.
Gasses have indefinite shape and volume.
You have a liquid with a density of 1.20 g/cm³ and another liquid with a density of 0.85 g/cm³. If you mix these two liquids, which one will be on top and which will be on the bottom? Explain your answer based on their densities.
The liquid with a density of 1.20 g/cm³ will be on the bottom because it is denser. The liquid with a density of 0.85 g/cm³ will float on top because it is less dense.
This particular object has a mass of 5.4 g and a volume of 2cm3, based on the chart which object is it?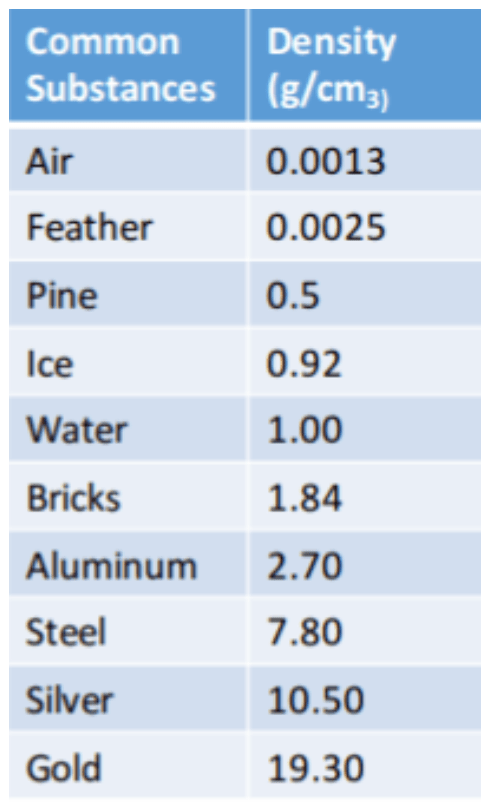
Aluminum
 Which element would be a Nonmetal?
Which element would be a Nonmetal?
D
Metalloids are...
A) Shiny, Malleable, Conductors
B) Dull, Brittle, Insulators
C) Shiny or Dull, Brittle, Semiconductors
D) Dull, Malleable, Conductors
C
What type of energy is applied to turn a water into water vapor?
Bonus (Extra 500) : When I heat up a liquid what is happening to the atoms?
Heat or Thermal Energy
When you heat up the liquid, the atoms start moving faster and faster.
Imagine you have three different liquids:
- Liquid A: Density = 0.95 g/cm³
- Liquid B: Density = 1.10 g/cm³
- Liquid C: Density = 0.85 g/cm³
You pour these liquids into a clear glass, one after the other, without mixing them.
Which liquid will be on the top, which will be in the middle, and which will be on the bottom?
- Liquid C (0.85 g/cm³) will be on the top because it has the lowest density.
- Liquid A (0.95 g/cm³) will be in the middle because its density is between Liquid C and Liquid B.
- Liquid B (1.10 g/cm³) will be on the bottom because it has the highest density.
In a mixture of liquids with different densities, the liquid with the lowest density floats on top, while the one with the highest density sinks to the bottom.
The following table provides the density of four different liquids. Rank the liquids from highest to lowest density and then determine where water should be added based on the density comparison.

- Mercury: 13.53 g/cm³
- Maple Syrup: 1.37 g/cm³
- Water: 1.00 g/cm³
- Olive Oil: 0.92 g/cm³
- Ethanol: 0.79 g/cm³
 The following properties describe which periodic table classification? -Good conductor of heat and electricity -Malleable -VERY reactive
The following properties describe which periodic table classification? -Good conductor of heat and electricity -Malleable -VERY reactive
Blue Elements - Metals
Where are the rare earth elements in the periodic table?
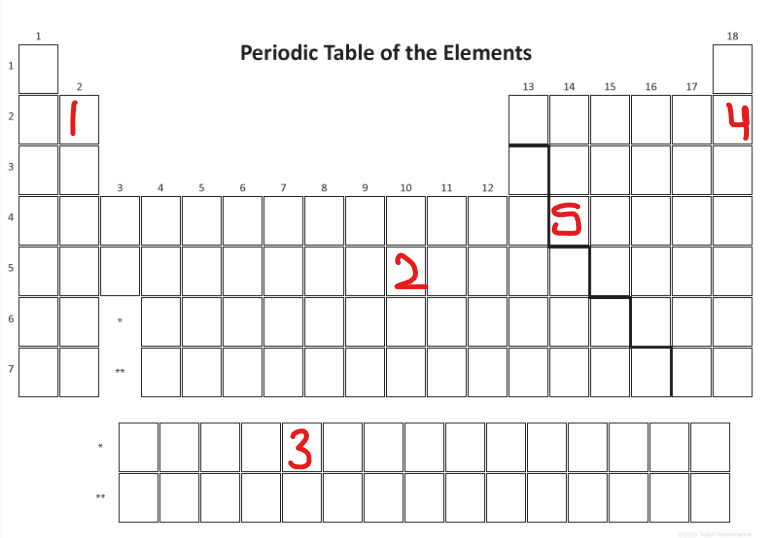
3