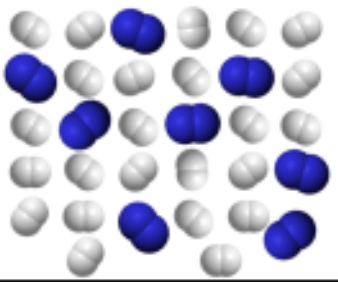
 Does this image illustrate an Element, Compound or Mixture?
Does this image illustrate an Element, Compound or Mixture?
M I X T U R E
Which of these distance measurements (given in scientific notation) is the shortest distance scale measurement?
a) 1.2 x 10-3 m
b) 5.8 x 10-6 m
c) 7.1 x 108 m
d) 9.3 x 101 m
b) 5.8 x 10-6 m
Round each number to the indicated precision.
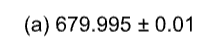
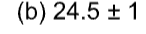
A) 680.00
B) 25
How many significant figures are there in each measurement below?
a) 0.00628
b) 1.5 x 106 mL
c) 62.00 s
d) 59.010 g
a) 3
b) 2
c) 4
d) 5
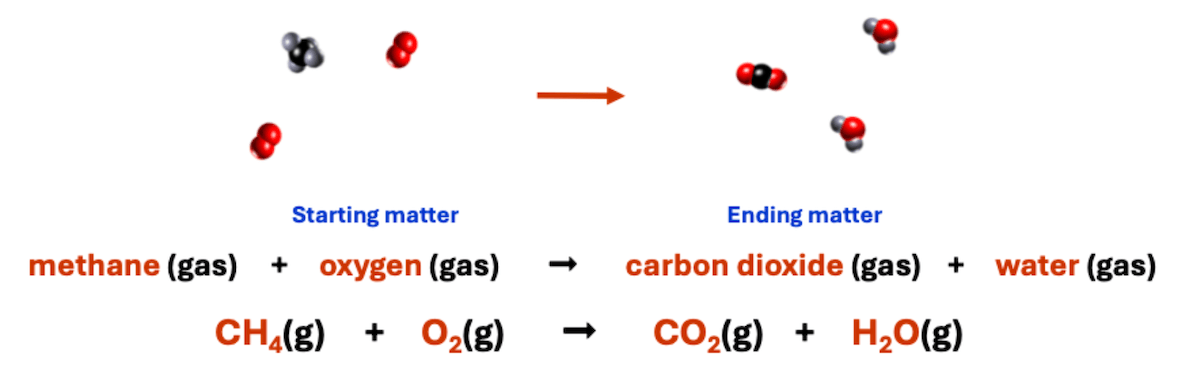
What type of change process does this chemical equation represent?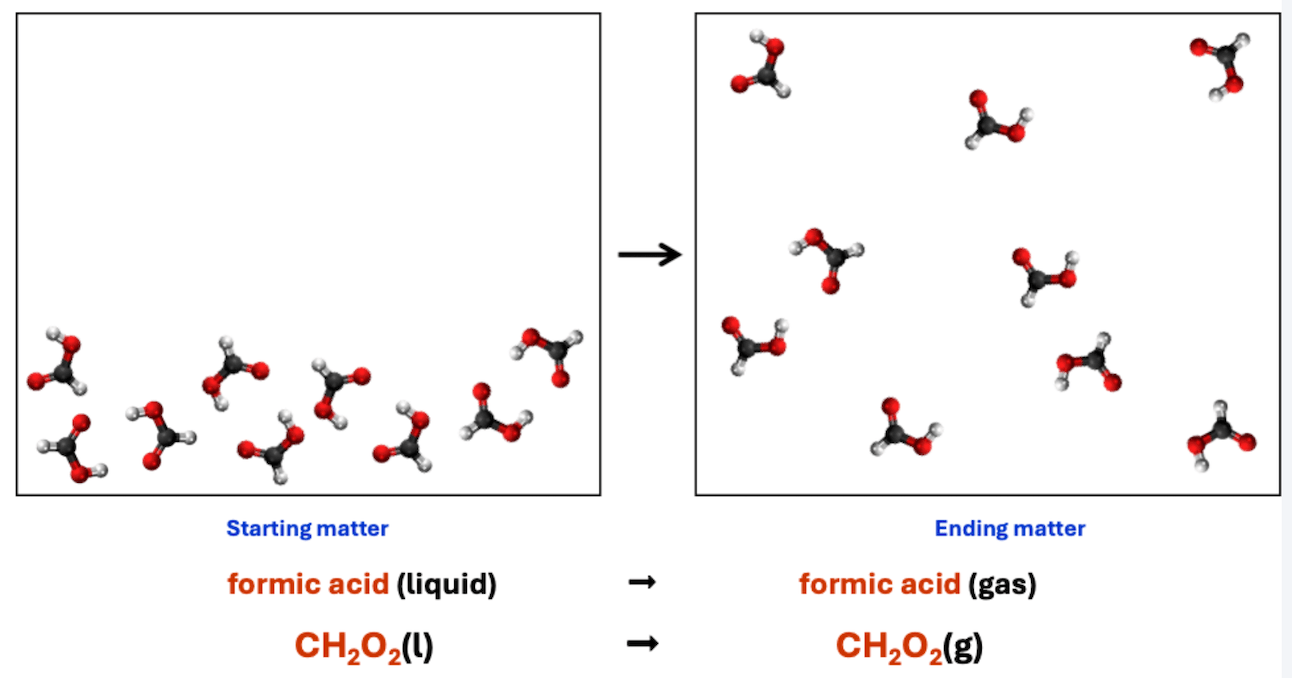
physical change
Identify each observation as either a chemical or physical property:
A) Yellow powdery solid substance.
B) Substance melting at 649 degrees celsius
C) Density of the substance is 1.738 g/cm
D) The substance reacts with chlorine gas to yield white brittle solid.
A) physical property
B) physical property
C) physical property
D) chemical property
What kind of change process does this chemical equation represent?
a) physical change process
b) both chemical and physical change processes
c) neither a chemical nor a physical change process
d) chemical change process
d) chemical change process
What are the names and unit symbols for the SI base units listed below?
A) Length
B) mass
C) time
D) temperature
A) meter, m
B) kilogram, kg
C) second, s
D)Kelvin, K
Perform the following conversions between Sl units.
a) 4 ug = _____ g
b) 0.13 mL = _____ cL
a) 0.000004
b) 0.013
93411 x 0.00335 = _____
What is the answer in the correct number of significant figures?
313
Identify each of the following as a pure substance, a homogenous mixture, or heterogenous mixture
A) Apple Juice
B) Pizza
C) Baking soda (sodium bicarbonate)
D) Milk (from grocery store)
A) Homogenous Mixture
B) Heterogenous Mixture
C) Pure Substance
D) Homogeneous Mixture
Which of the following chemical equations represents a physical change process?
a) C3H8 (g) -> C3H8 (l)
b) Al (s) + O2 (g) -> Al2O3 (s)
c) N2 (g) + O2 (g) -> N2O4 (g)
d) C (s) + Cl2 (g) -> CCl4 (l)
a) C3H8 (g) -> C3H8 (l)
Change the unit used to report each of the following measurements by replacing the power of ten with the corresponding SI prefix.


A) 4.54 ng
B) 3.76 km
C) 1.8 cL
D) 6.34 µg
Classify each student’s values according to their accuracy and precision.
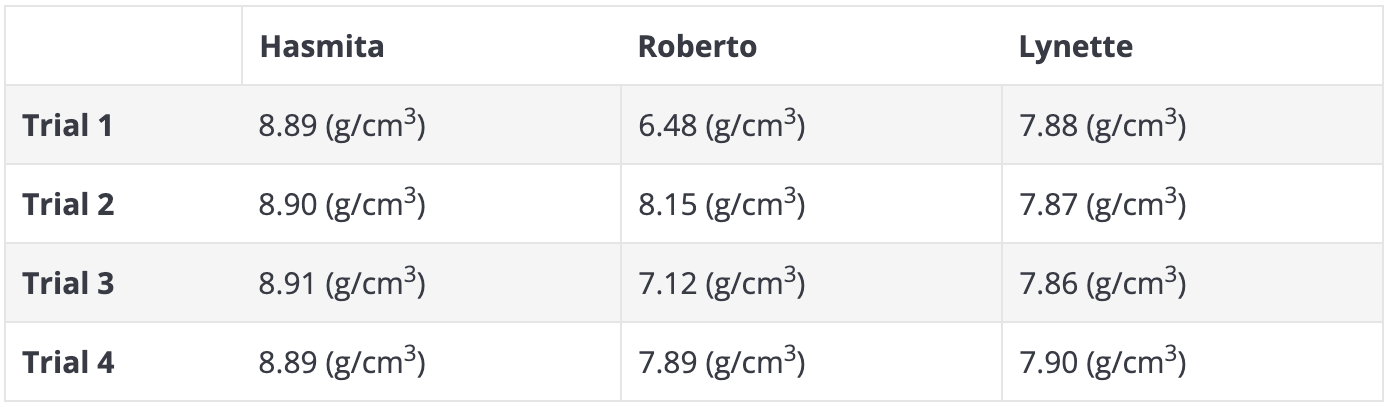
a) Hasmita's values are _____
b) Roberto's values are _____
c) Lynette's values are _____
d) Which student's values show systematic error?
a) precise but not accurate
b) neither precise nor accurate
c) both accurate and precise
d) Hasmita
Bromine is a brownish red liquid with a density of 3.10 g mL −1 . What volume would a 88.5 g sample of bromine occupy?
28.5 mL
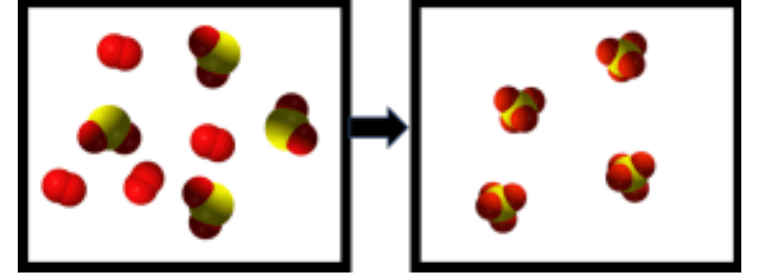
Is this a Chemical or Physical Change?
- Explain your answer :PP
This is a chemical change!!!!
Your tell-all here is the rearrangement of atoms and the breaking and formation of chemical bonds!
What is the primary way of thinking about energy that you are using when you say the following statement?
"I put gasoline in my car so that the car's engine can get energy to go."
Energy is casual
Perform the following unit conversions!

A) 1.55*10^2 kg * (1000 g /1 kg) * (1000 mg / 1g)
= 1.55e8 mg
C) 87.4 cm^3 *(10^3 / 1^3)
= 8.74e4 mm^3
Perform the calculation below and select the answer with the correct significant figures.
3.5 cm x 4.217 cm x 5.36 cm = _____
a) 79
b) 79.1
c) 79.11
d) 79.111
a) 79
Classify each of the following substances as a/an element, compound, homogeneous mixture, or heterogeneous mixture.
a) sucrose (C12H22O11)
b) granite
c) 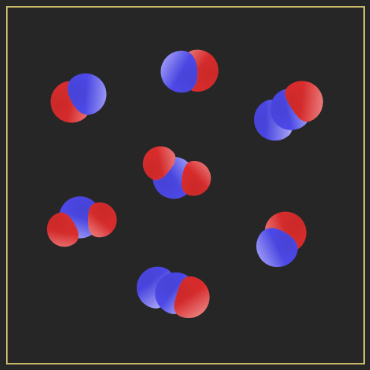
d) 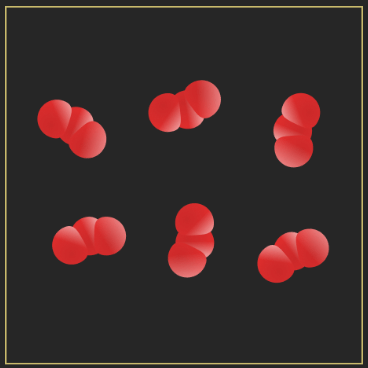
a) compound
b) heterogenous mixture
c) homogenous mixture
d) homogenous.
State whether each of the following is a pure substance, element, compound, molecule, molecule & compound, or mixture.
A) Ag
B) CO
C) CaCO3
D) O2
A) Element
B) Molecule/Compound (Molecular Compound)
C) Molecule/Compound (Molecular Compound)
D) Molecule NOT a Compound
Classify each of the following substances as a/an element, compound, homogenous mixture, or heterogeneous mixture.
a) red wine
b) sulfur (S8)
c) 
d) 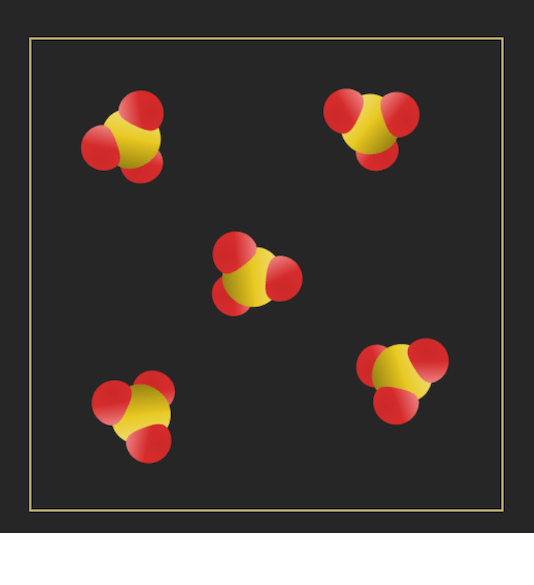
a) homogenous mixture
b) element
c) heterogenous mixture
d) compound
An empty 25-mL volumetric flask has a mass of 25.6425 g. Once filled with diethyl either, the flask has a mass of 43.4815 g. What is the density of the diethyl ether? (Note: Volumetric glassware is very precise, so you may assume a precision of ± 0.01 mL for the volume)
D = M * V
(43.4815 g - 25.6425 g) / 25.00 mL
D = 17.8390 g / 25.00 mL
D = 0.71356 g/mL-1. OR 0.71? or 0.714?
You use a balance and find that the mass of the piece of metal is 56.72 g. To determine the volume of the metal, you fill a graduated cylinder to 31.0 mL and then drop the piece of metal into the graduated cylinder.
The final volume is 38.9 mL. What is the density of the piece of metal?
7.2 g/mL
A particularly hot summer day is 19.5 degrees fahrenheit. What is this temperature in celsius?
43.1