3 things you need to wear to each lab.
What is long pants, closed-toe shoes, and your apron/smock.
What is a problem statement?
The three primary base units of the metric system.
What are meter, liter, and gram?
The system of measurement used by scientists internationally.
What is the metric system?
The state of matter that has no definite shape, but does have definite volume.
What is a liquid?
A way to hold a test tube solution when heating it.
What is with tongs, and facing away from you and your lab partner.
Term that uses your senses as a way to conduct research for the experiment.
What is making an observation?
6 mm = ___________ m
What is 0.006 m ?
 State whether these darts represent accuracy, precision, both, or neither.
State whether these darts represent accuracy, precision, both, or neither.
What is precise?
A solute in the mixture of a milkshake.
What is milk/ice cream/sugar/flavoring?
A way to immediately get a referral during a lab.
What is horseplay?
The variable that relies on the independent variable.
What is the dependent variable?
0.382 dm = _______ mm
What is 38.2 dm?
Convert 3,400,000 from standard notation to scientific notation.
What is 3.4 x 106?
The type of separation technique used to separate homogenous mixtures.
What is distillation?
The glassware used to measure 17 mL of solution.
What is a graduated cylinder?
The belief of something that is on the cusp of becoming science, yet is not testable.
What is pseudoscience?
23 kg = __________ mg
What is 23,000,000 mg?
Solve the equation in scientific notation.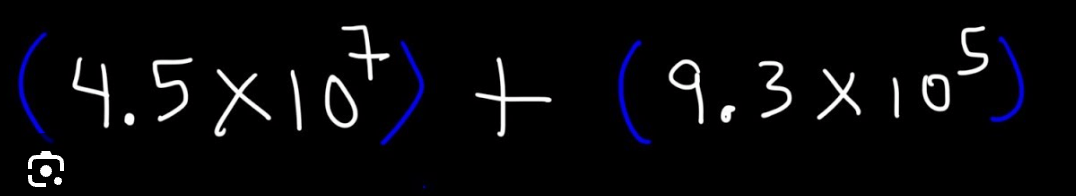
What is 4.593 x 107?
What type of property is the following: color
What is a physical property?
The lab equipment that is used for measuring mass.
What is a triple beam balance/digital scale?
Each of the 7 steps of the scientific method.
What is problem statements, ask a question, background research, form hypothesis, experiment, draw a conclusion, and report results.
78.34 cL = _________ hL
What is 0.007834 hL?
Solve the following in scientific notation:
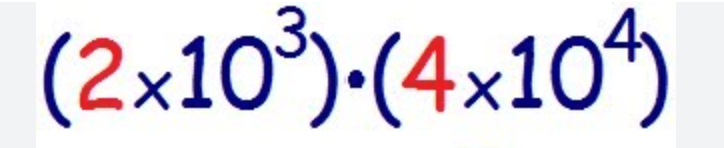
What is 8 x 107?
The two types of physical properties.
What are extensive and intensive properties?