Name the components of the scientific method.

Observation, hypothesis, Experiment, Data Collection, Conclusion
Color, Boiling Point and Luster are all examples of:

Intensive Properties
Explain the process of either distillation or filtration:
I'll come around!
2.3x102 cm = _____ m
100cm = 1m

2.3 m
When graphing the ______ variable goes on the x axis.
What are our five senses we use to make observations?
Taste, smell, touch, sight, hear
Ice melting, Water Freezing and Water Boiling are all examples of:

Physical Changes
An experimental measurement was taken of 10.4mL and the actual measurement was 9.7 mL. What is the percent error?
7.2%
62.8 L= ___deciliters
10 dL = 1 L
628 dl
During a chemical reaction, the _______ composition of the starting substance changes.
chemical
A researcher is studying how length of sleep affects test scores. Label the dependent variable and the independent variable

Dependent: Test Scores
Independent: Length of sleep
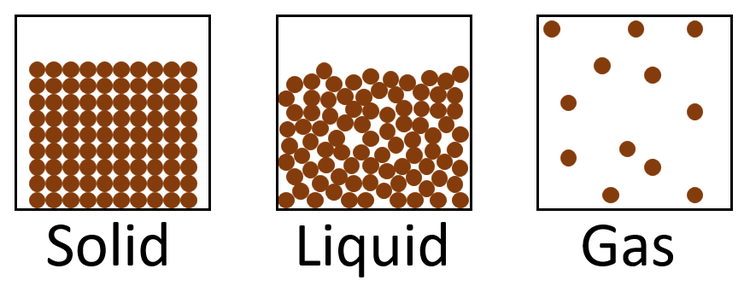
Gases, Liquids, Solids.
Put the phases above in order from the one with the closest particles to the one with the particles most spread apart. Draw a particle diagram for each proving your point.
solid, liquid, gas
A student estimated a mass to be 325000 mg. However, the actual mass was 342 g. What is the percent error?
1000mg=1g
4.9%
Put 0.00002389 in scientific notation
2.389x10-5
9.76x104= ________
97600
Draw a graph of the following data. Be sure to label everything!
Amount of water (mL): Plant height (in.)
10 mL 8 in.
15 mL 12in.
20mL 20in.
I'll come around!
Blood, Chicken broth and soda are all:

homogeneous mixtures
Explain why mass is a extensive property
The more AMOUNT of mass the more mass there is.
9.24x10-2 meters= _____mm
92.4 mm
When is a line graph a good option for graphing?
To see a increase/decrease trend over time.
If your hypothesis is proven false, you must have done the experiment wrong. True or False
False!
Name 5 things that are matter
Raise the boards up!
Which of the groups had the most precise data?
.png)
Group 2
524 mL = _____ kL
must be in scientific notation.
1000 mL=1L
1000 L = 1mL

0.000524 or 5.24x10-4
A student running 2 meters/second, is running _____ miles/hour
1609 meters= 1 mile
4.47 miles/hour