In which kingdom would yeast, which is a single celled organism, fall under?
A. Animalia
B. Plantae
C. Fungi
D. Protists
C. Fungi
A molecule that contains all polar covalent bonds can be considered:
A. Hydrophilic
B. Hydrophobic
C. Amphiphilic
A. Hydrophilic
Water is able to have surface tension due to:
A. Polar Covalent bonds
B. Nonpolar covalent bonds
C. Hydrogen bonds
D. Polarity
C. Hydrogen bonds
[OH-] = 1 x 10-8
Find:
[H+] =
pH =
A. [H+] = 6 pH = 6
B. [H+] = 1 x 10-6 pH = 8
C. [H+] = 1 x 10-420 pH =8
D. [H+] = 1 x 10-6 pH = 6
D. [H+] =1 x 10-6 pH = 6
The following is an example of which driving force of evolution?
A dog breeder is trying to breed teacup Shi-Tzu's, so that he can have more teacup Shi-Tzu's to sell. He purposely chooses teacup Shi-Tzu's to mate with other teacup Shi-Tzu's.
A. Natural selection
B. Artificial selection
C. Mutations
B. Artificial selection
Plants are known as producers. If they make their own food, what else could they be classified as?
A. Heterotrophs
B. Consumers
C. Heteroautrophs
D. Autotrophs
D. Autotrophs
All of these are the four elements that make up 96% of the human body except for:
A. Nitrogen
B. Carbon
C. Phosphorus
D. Oxygen
E. Hydrogen
C. Phosphorus
This water molecule has four hydrogen bonds, the water is most likely in which state of matter?
A. Gas
B. Liquid
C. Solid
D. Plasma
C. Solid
Find the acid & the base in the reactants:
Li2H3 + H2O -> Li2H4+ + OH-
A. Acid: Li2H3 Base: H2O
B. Acid: H2O Base: Li2H3
C. Base: Li2H3 Acid: OH-
D. Base: H2O Acid: Li2H4+
B. Acid: H2O Base: Li2H3
Pentane and isopentane have the same number of carbons and the same number of hydrogens (C5H12). However, they are structured differently. This is an example of...
A. Saturated fat
B. Unsaturated fat
C. Chemical bond
D. Isomer
D. Isomer
Rabbits that live in a forest in Canadia are either black or brown. Suddenly a white rabbit was born during heavy winter snow. That white rabbit had more little white baby rabbits. The black and brown rabbits were being eaten, while the white rabbits camouflaged in the snow.
If things keep going this way, the driving force behind evolution would be:
A. Natural selection
B. Artificial selection
C. Mutation
D. None of these
C. Mutation
The chemical bonds that hold NH3 would be considered
A. Intermolecular
B. Intramolecular
C. Hydrogen Bonds
D. Nonpolar
B. Intramolecular
After studying for the first biology 189 test, I decide to drown my sorrows with some kool-aid. I pour water in a container, then I put the kool-aid powder, finally I stir it with a spoon. The kool-aid is the...
A. Solvent
B. Solution
C. Compound
D. Solute
D. Solute
The normal pH for a certain bird species is 7.5 pH, but after consuming food its pH goes up to 7.7
True or False: Buffers will seek to neutralize the pH of the bird.
A. True
B. False
B. False
Buffers stabilize pH, it will not neutralize it. If the pH went down to 7 (neutral), the bird would die
Name the functional group:
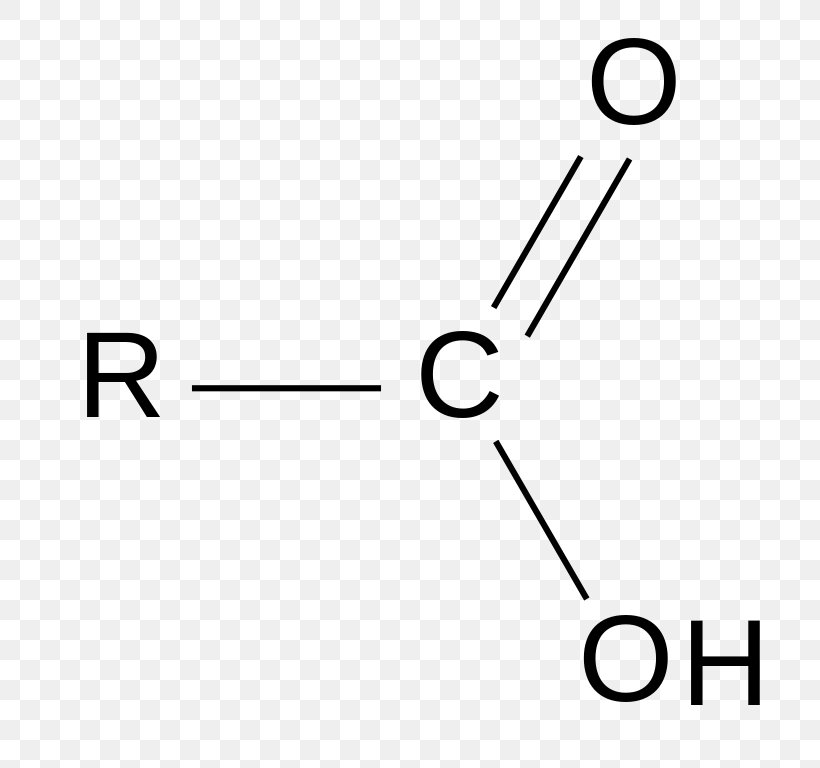
A. Methyl
B. Carbonyl
C. Carboxyl
D. Hydroxyl
C. Carboxyl
A chad microbiologist uses a microscope to look through a slide containing single celled organisms that have a nucleus. What organism is the chad microbiologist most likely looking at?
A. Bacteria
B. Protist
C. Archaea
D. An animal cell
B. Protist
Lithium (Li) has one valence electron and Chloride (Cl) has seven valence electrons.
What type of bond would they form?
Who would be the cation?
A. Bond: Covalent Cation: Cl
B. Bond: Covalent Cation: Li
C. Bond: Ionic Cation: Cl
D. Bond: Ionic Cation: Li
![]()
D. Bond: Ionic Cation: Li
When boiling water, the heat energy is first disrupting the...
A. Covalent bonds
B. Hydrogen bonds
C. Hydrogen atoms
D. Intramolecular bonds
B. Hydrogen bonds
1. In a neutral solution, I increase the number of hydroxide ions. The pH would
2. In a neutral solution, I decrease the number of hydronium ions. The pH would
A. 1. Increase, 2. Decrease
B. 1. Decrease, 2. Increase
C. 1. Increase, 2. Increase
D. 1. Decrease, 2. Decrease
C. 1. Increase, 2. Increase
Li+ & Cl- are put in a container in which water is the solvent. What happened to the Li+ & Cl- after I stirred them?
A. The partial positives and negatives formed hydrogen bonds
B. The partial positives of hydrogen surrounded Li
C. The partial negatives of oxygen surrounded Li
D. The partial positives of hydrogen surrounded Oxygen
C. The partial negatives of oxygen surrounded Li
Name the 7 properties of life & give a brief overview of each.
Order - Organized. Nothing is randomly assorted.
Regulation - Maintain balance like homeostasis
Reproduction - Making offspring by a sexual or asexual process
Energy processing - Consuming "food" from the environment & converting it into energy
Growth & development - Being able to grow and mature
Response to the environment - Feeling hot or feeling cold
Evolutionary adaptation - Adapt to the environment; takes many generations
Name all the bonds & briefly describe each one
Covalent - Share electrons & can have polar covalent or nonpolar covalent bonds
Ionic - Transfer electrons & opposite charges attract
Hydrogen - The partial positive hydrogen from a polar molecule attracts to the partial negative atom of another polar molecules
Van der Waals - Attractions between two uncharged molecules that come close together due to temporary charges
Hydrophobic interactions - Nonpolar substances will group up together when exposed to water
Name the four properties of water & give a brief explanation of each
Cohesion & Adhesion - Water can stick to itself (cohesion) and water can stick to anything else (adhesion)
Moderation of temperature - Water can resist temperature changes
Expansion upon freezing - Molecules are more spread when water is in ice form
Versatility as a solvent - A lot of substances can dissolve in water
You have 2 buffers in your body:
H2CO3 (carbonic acid) & HCO3- (bicarbonate base)
You ate too many oranges because oranges = helth
![]()
Oranges cause an increase in hydrogen ions (H+). How will your body get rid of those hydrogen ions?
By getting rid of hydrogen ions, which of the two buffers will increase?
Your body will get rid of those hydrogen ions by releasing HCO3- (bicarbonate). H+ will attach to HCO3- turning it to H2CO3
Because HCO3- is being turned into H2CO3 the carbonic acid H2CO3 is increasing.
Name the three domains of life & all the kingdoms that correspond to their domain of life.
Domains: Bacteria, Archaea, Eukarya
Kingdoms in Bacteria: Bacteria
Kingdoms in Archaea: Archeae
Kingdoms in Eukarya: Protists, Animalia, Plantae, Fungi