What is the acronym for using the fire extinguisher? Spell each word out.
P: Pull the pin
A: Aim the extinguisher at the fire.
S: Squeeze the trigger
S: Sweep side by side 8-10 feet away from fire
What is the following lab equipment called?
Graduated Cylinder
What tool do we use to measure length in the lab?
Ruler/Meter Stick
Where do scientists publish their results?
Scientific Journal/Periodical
How many protons are in Argon?

18

to put out small fires.
What is this tool called?

A triple beam balance
What type of system of measurement do scientists use, as well as the major part of the world (except United States of America)?
Metric system
What is the name of process where scientists heavily edit other scientists work?
Peer Review
How many neutrons are in Gold (Au)?
118 neutrons
What is a corrosive substance?
A corrosive substance will damage or destroy other substances with which it comes into contact by means of a chemical reaction. It has the ability to attack and potentially destroy body tissue.
What is a petri dish used for?
Used to grow bacteria and fungi
What is the formula to calculate density?
What is the difference between scientific law vs. theory?
A scientific law attempts to DESCRIBE a scientific phenomenon.
A theory attempts to EXPLAIN a scientific phenomenon.
What is the difference between kinetic and potential energy? Give an example of each.
Kinetic energy means energy dealing with movement. Potential energy is stored energy.
Kinetic energy examples: throwing a ball, a racing car
Potential energy: Car on top of a hill, water reservoir behind dam.
What does this symbol mean?
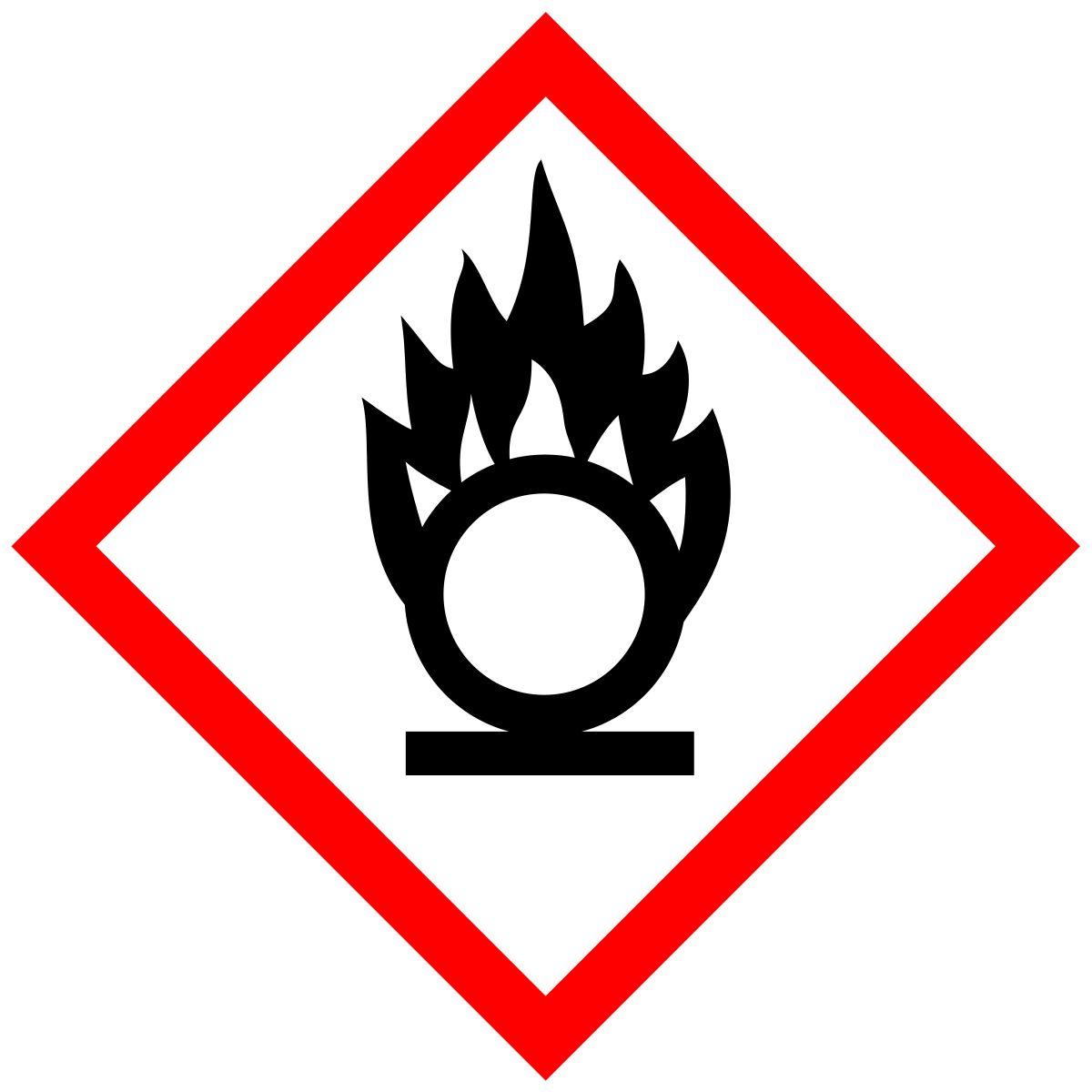
Oxidizer, severe fire hazard and promotes combustion in other materials.
What is this piece of equipment AND what is its function?
Erlenmeyer flask.
Used to measure and contain liquid, particularly useful to gently stir the content inside the liquid as it has a narrow neck and slanted sides.
A student needs to determine the density of an unknown rock sample by dropping the rock into
a graduated cylinder containing water. The original volume in the graduated cylinder is 20.0
mL. The student then drops the rock sample into the graduated cylinder and determines the
new volume to be 30.0 mL. If the mass of the rock is determined to be 18 grams, what is the
density of this sample?
1.8 g/mL
What are the 6 steps to the Scientific Method (also called Experimental Design) process?
1: Make observations
2: Ask a question
3: Form a hypothesis
4:: Test hypothesis by conducting experiment
5: Collect and Analyze Data
6: Draw a conclusion
What is the atomic number and atomic mass in Tm? AND calculate how many protons, electrons, and neutrons there are.

Atomic Number: 69
Atomic mass: 169
Protons: 69
Electrons: 69
Neutrons: 100
What does this chemical symbol mean?
A corrosive substance.
What is this equipment?

a compound light microscope.
Why does oil float on top of water? Hint: think about density
Oil is LESS dense than water. Anything that has a density LESS than water, will float. Anything that has a density MORE than water, will sink.
Recall Francesco Redi's Experiment.
What was the independent variable?
What was the dependent variable?
What were some constants?
What was the control group?
What was the experimental group?
Independent variable: covering of the jar
Dependent variable: number/presence of maggots.
Constants: type of jar, environment, type of meat, etc.
Control group: jar with no covering
Experimental group: jar with covering
What is the following law called:
Whenever matter undergoes a physical or chemical change, no atoms are created nor destroyed
Law of Conservation of Matter