(periodic table needed)
What do Solids, liquids and gasses and plasma look like on the molecular level in terms of density?
From most dense to least, solid, liquid, gas and plasma
What elements bond in a ionic bond?
Metal and non-metal
What is a combination reaction?
X + Y → XY
24.31+16.00=40.31g/mol
Sodium Bromide
What is a crystalline solid?
a solid that has a crystal lattice
What elements typically bond in a covalent bond?
non-metal and non-metal
What is a decomposition reaction?
AB->A+B
What is the difference between molar mass and atomic mass?
molar mass=g/mol is weight of LOTS of atoms
atomic mass=amu is mass of one atom
P2O5
diphosphorous pentoxide
What is an amorphous solid?
non-crystalline, non-rigid structure, does not have a defined lattice pattern.
How do we find the charge of transition metal ions from the chemical formula?
criss-cross method
What is a double replacement reaction?

How many moles are in 50 g of O?
The molar mass of oxygen is 15.999 g/mol.
50g O×1molO/15.999g O=3 mol O
silicon dioxide
SiO2
define homogenous and heterogenous mixture.
A homogeneous mixture is a mixture in which the composition is uniform throughout the mixture.
A heterogeneous mixture is a mixture with a non-uniform composition. The composition varies from one region to another with at least two phases that remain separate from each other, with clearly identifiable properties.
Molecules are held together in a crystal by
Van der Waal's attraction
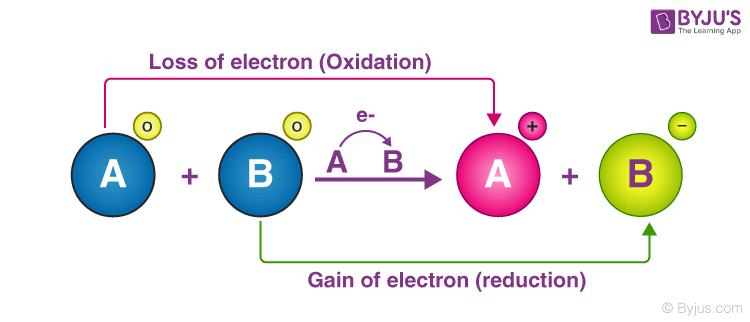
What is a redox (reduction-oxidation) reaction?
50g of Vanadium is mixed with 50g of Ozone (O3) to produce 65g of Vanadium (V) Oxide. What is the percent Yield? Hint: this is the unbalanced formula V+O3-> V2O5
72.8%
Diboron tetrabromide
B2Br4
What are the 5 types of chemical changes?
The five basic types of chemical reactions are combination, decomposition, single-replacement, double-replacement, and combustion.
Ionic bonds easily form when electron when ionization energy of the metallic atom is _____ comparatively.
less
Why do we call it a redox (reduction-oxidation) reaction instead of a reduction reaction or an oxidation reaction?
Because oxidation and reduction usually occur together.
89.2650g of Vanadium is mixed with 50g of Ozone (O3) to produce 65g of Vanadium (V) Oxide. What is the theoretical yield? Hint: this is the unbalanced formula V+O3-> V2O5
89.26
Tin(IV) selenide
SnSe4