Based on the following chemical model below, the farthest circle (third energy level) contains what kind of electrons?
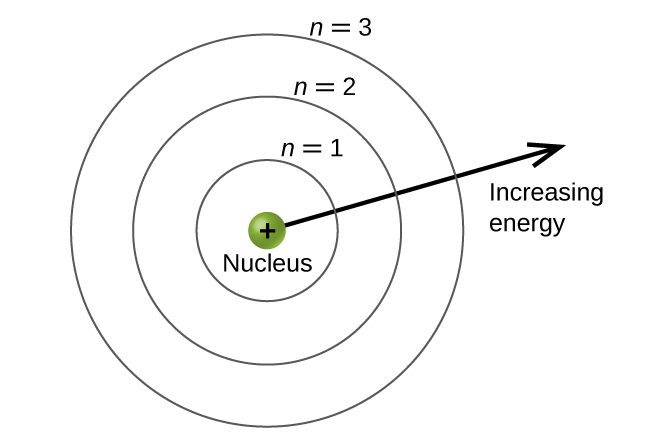
Valence Electrons
Name the following compound and determine if it's ionic or covalent?
BaCl2
Barium Chloride
Ionic - Metal and Nonmetal
Elements in group 2 has how many valence electrons? What is the group name of these elements?
2 valence electrons, Alkaline Earth Metals
Lewis dot structures only show these types of electrons
valence electrons
intermolecular forces exist between ________ compounds.
covalent

Based on the following picture of the movement of an electron, from the second to the first energy level, what happens to the energy? (Released or Absorbed)
Since the electron goes from a high to a low energy level, energy is being is released!
How can you tell if a compound is ionic?
If the compound contains a metal and a nonmetal and they are oppositely charged, this is considered as an ionic compound.
Rank the following atoms from smallest to largest in terms of their atomic size:
Li, H, Fr, Cs
H < Li < Cs < Fr
Draw the Lewis structure for water

order the 3 intermolecular forces we learned about from strongest to weakest
hydrogen bonding, dipole-dipole, London Dispersion Forces
If you compare two atoms and you found out that they are "isotopes," what features do they have in common? What features do they differ?
Same number of protons, different number of neutrons
P2F5
Covalent (Only nonmetals)
What is the most electronegative element in the periodic table?
Fluorine!!!!
The following diagram shows the transfer of electrons to form what ionic compound? (Please provide the chemical name/formula)
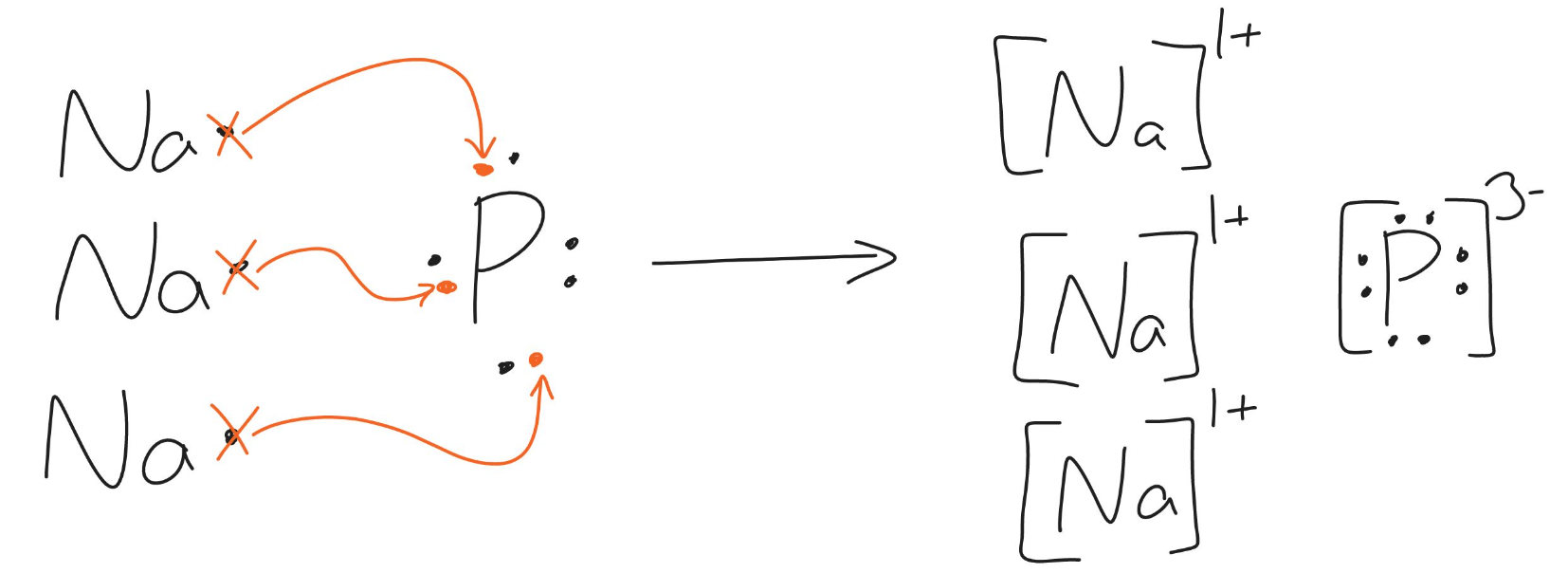
Sodium Phosphide (Na3P)
fluorine, oxygen, and nitrogen are 3 elements that are necessary for the formation of these IMFs
hydrogen bonds
Chlorine-38 (Atomic number: 17) has how many neutrons?
# of Neutrons: Mass Number - Atomic Number
38 - 17 = 21 neutrons
Calcium reacts with Fluorine to form Calcium Fluoride. What is the chemical formula of this compound?
Ca2+ + F- --> CaF2
For every two atoms of fluorine there is 1 atom of calcium!!
Which of the following elements have similar characteristics with one another? Why?
Ca vs. Ba or S vs. Cl
Draw the lewis dot structure for CaCl2
This is an ionic compound, therefore, you will need to include brackets on the drawing.
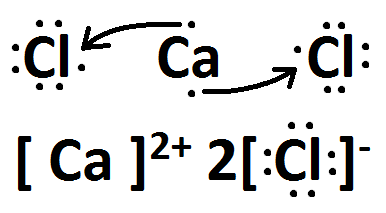
CH3Cl is considered to be a polar covalent molecule, what is the strongest intermolecular force does this molecule contain?

This molecule has dipole-dipole and London dispersion force, however, the strongest force this has is dipole-dipole.
Explain the difference of a molecule and an ionic compound:
Molecule: two or more nonmetallic atoms that share electrons to form a bond
Ionic Compound: two or more atoms (metal/nonmetals) transfers electrons to form a bond
Please provide two examples on what sets an ionic compound apart from a covalent compound.
An ionic compound has a stronger intramolecular attraction as compare to a covalent compound due to the high melting point.
In addition, ionic compounds can conduct electricity due to the presence of ions when melted/dissolved.
Which of the following elements have the same energy level with Boron? Why?
Neon, Aluminum, Helium, or Silicon
Neon, both of the elements are located in the same period (same row)
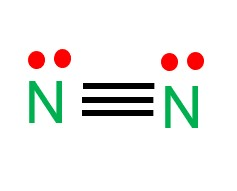
Draw the lewis structure for a nitrogen molecule
Linear
How do you know if a molecule is considered to be polar or nonpolar?
If the electrons are shared unequally in a molecule then it will be polar.
However, if the electrons are shared equally in a molecule, then it will be considered as nonpolar