Earliest recorded reference to atomic theory is attributed to this philosopher.
Democritus
the atom is composed of these three particles
protons, neutrons, electrons
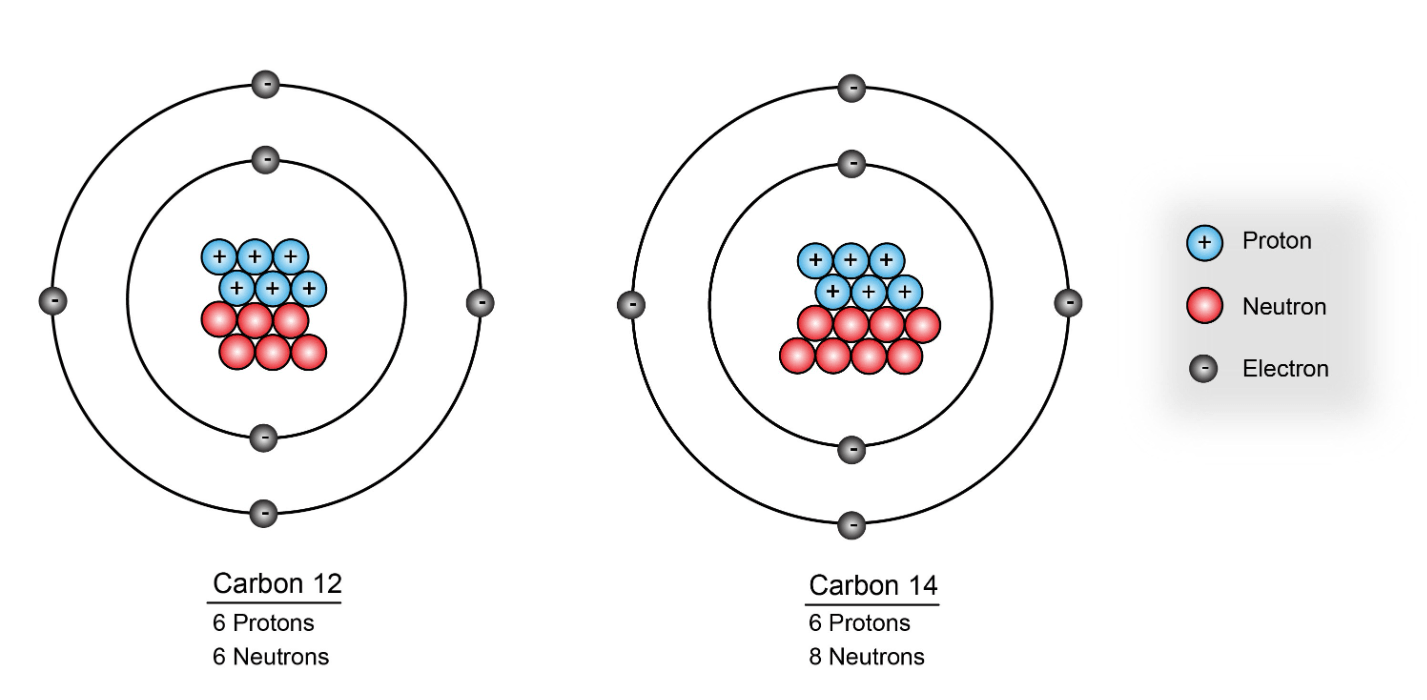
Isotopes are considered to be the SAME element.
(You get one shot)
True
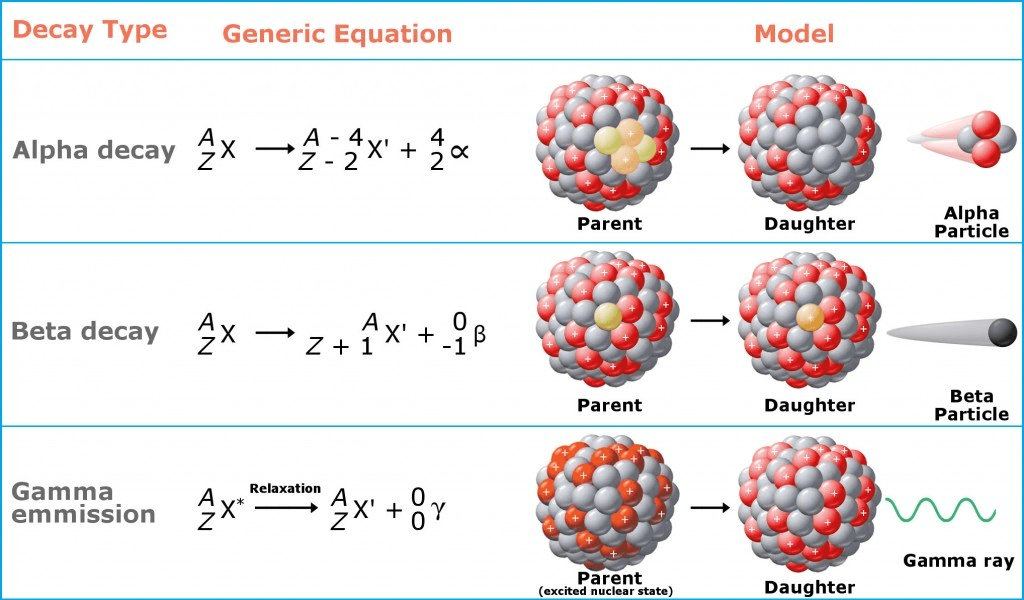
the spontaneous emission of rays or particles from the nucleus of an atom.
nuclear/radioactive decay
or radioactivity
He believed that matter was made of earth, water, air, fire, and aether.
Aristotle

These subatomic particles are positively charged
protons

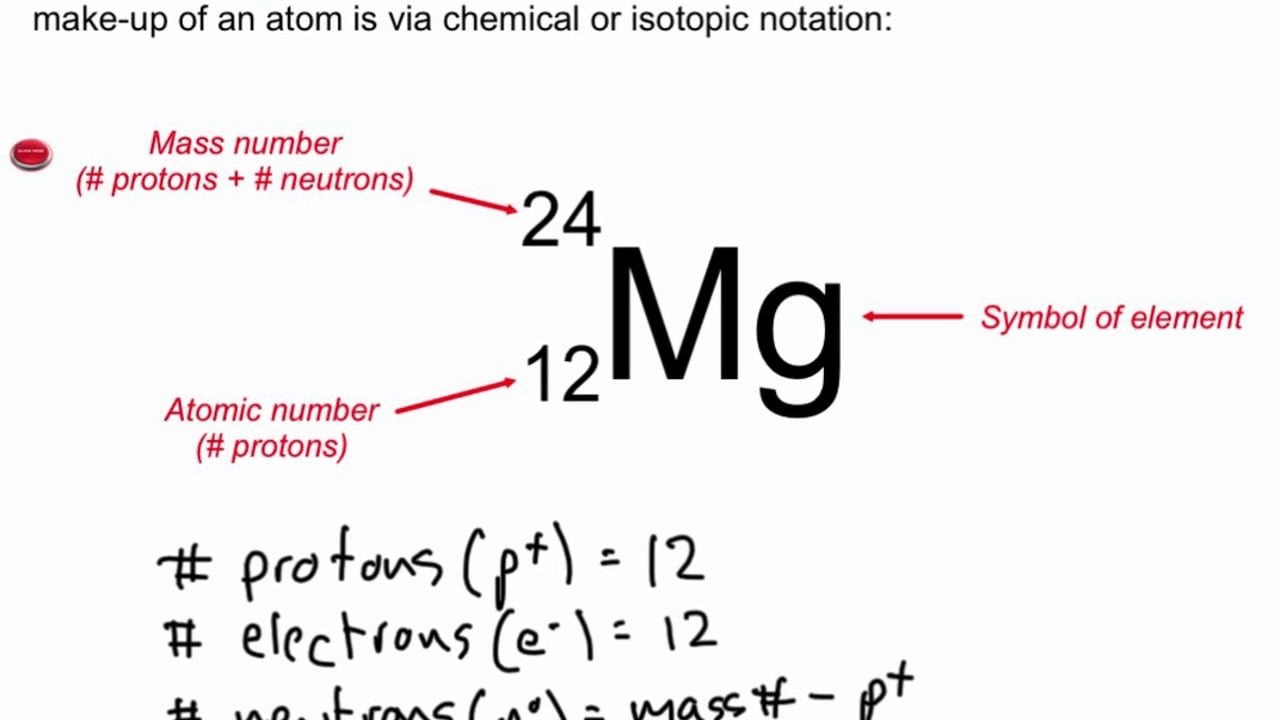
Mass number represents...
#protons + #neutrons
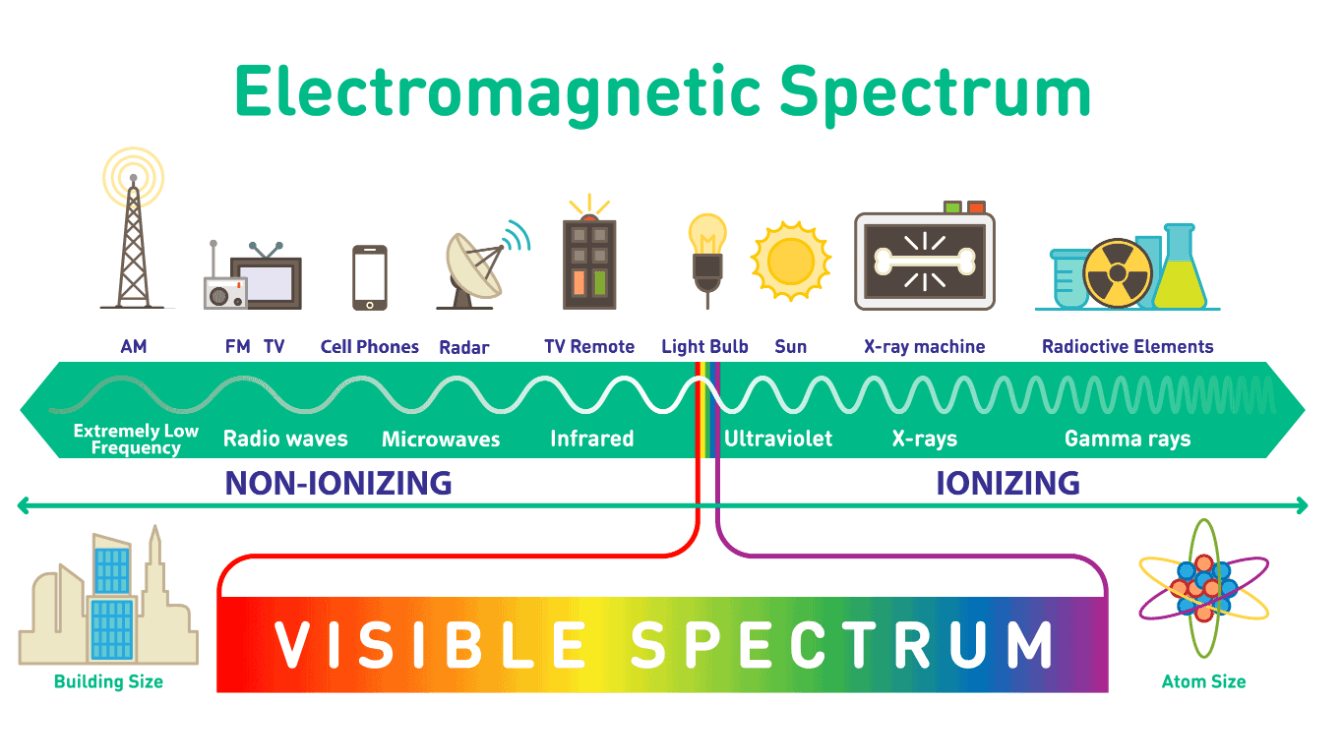
This radiation requires at least 4 meters of lead to block.
gamma radiation
This experiment determined the mass and charge of an electron.
Oil drop experiment
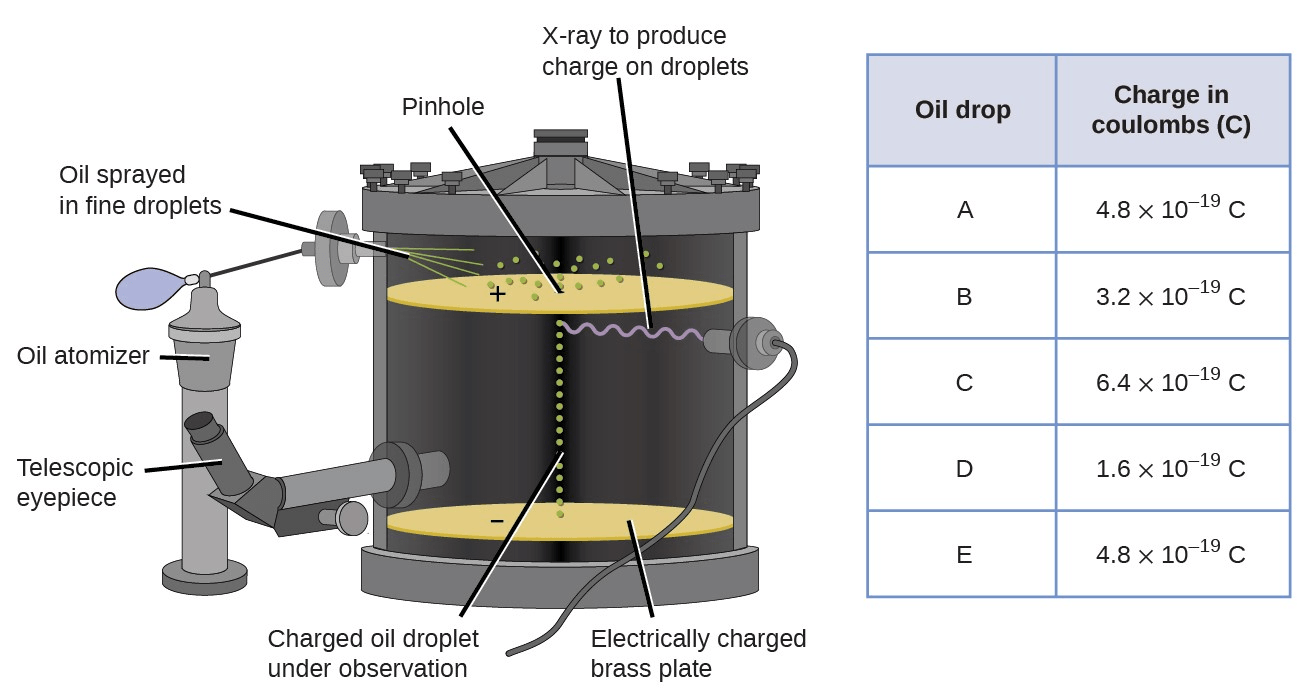
Over 99% of an atom's mass is located here.
core/nucleus
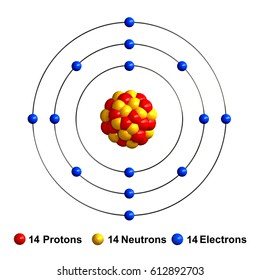
In isotope notation, the superscript at the top left of the symbol represents __.
mass number
Which type of radioactive decay does not produce a different element?
gamma decay

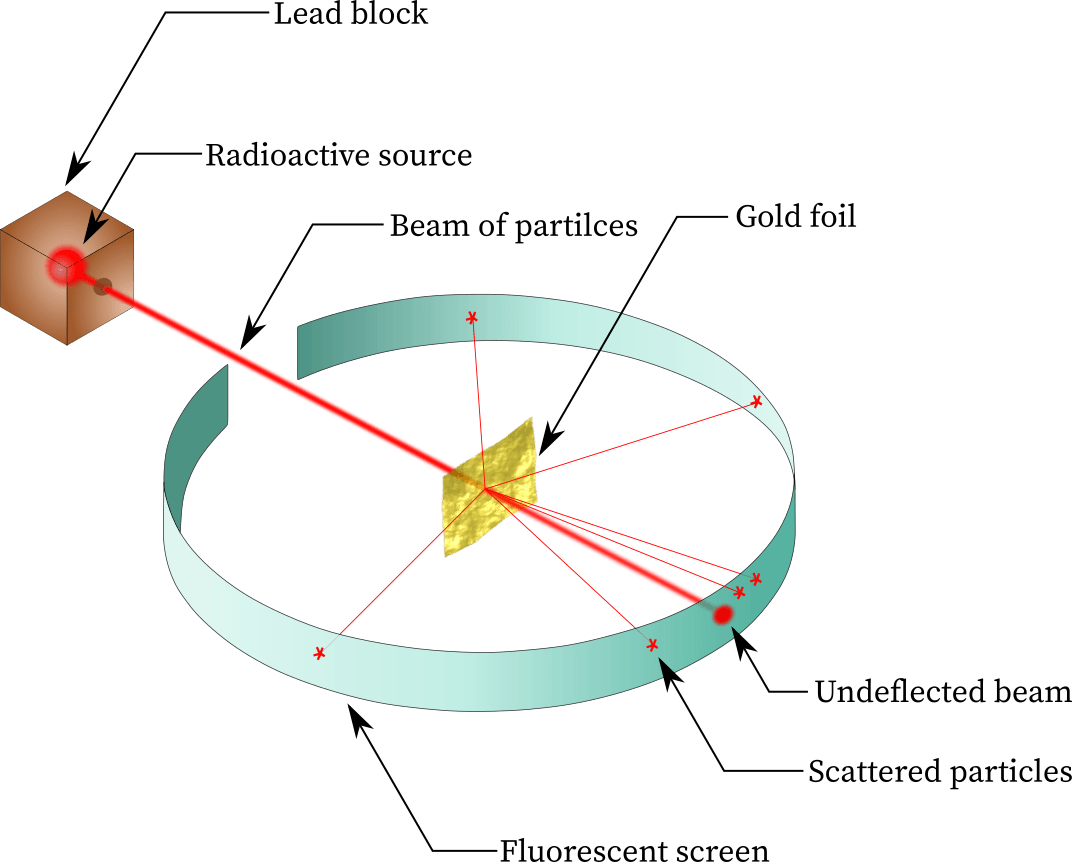
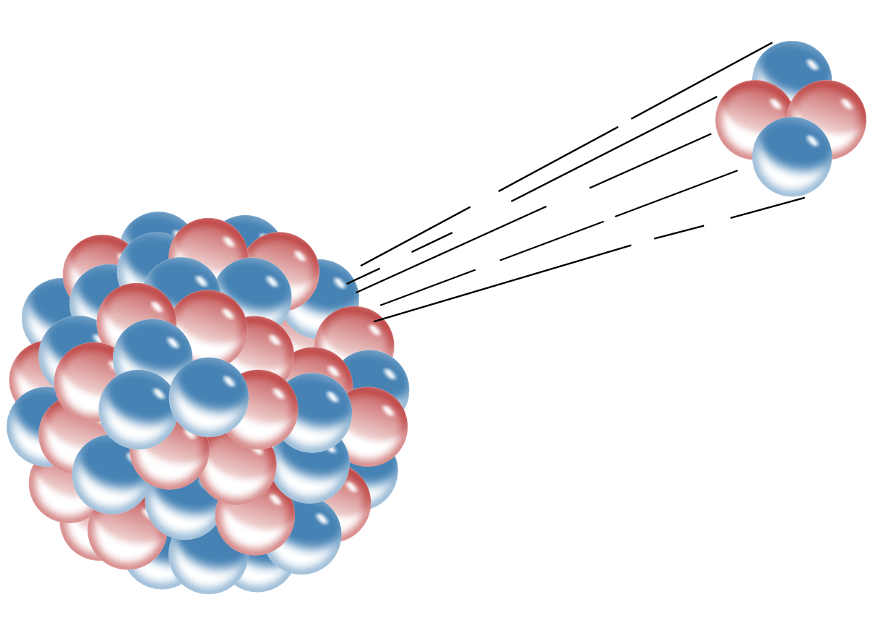
This experiment proved that an atom is mostly empty space with a dense positively charged core.
Gold foil experiment
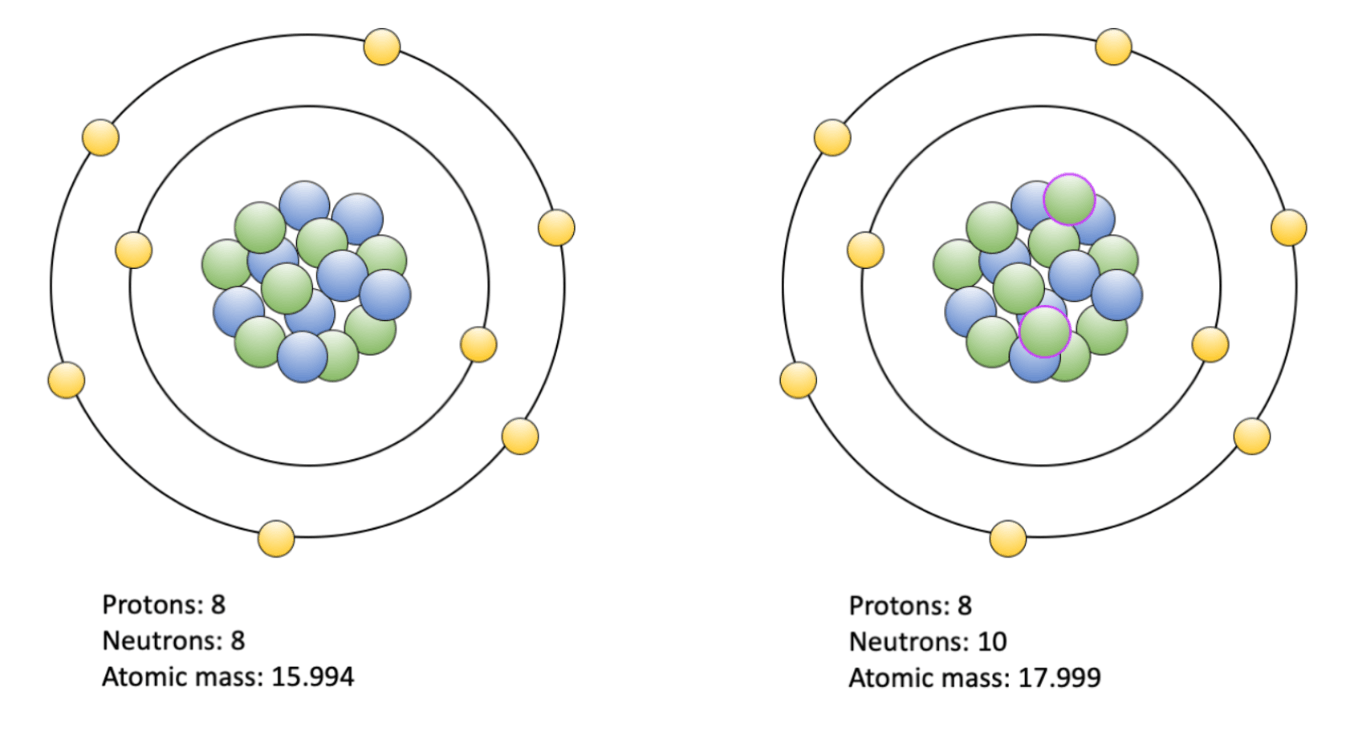
Atoms that possess the the same number of protons, but a different number of neutrons.
isotopes
Quantifies the occurrence of a particular isotope in nature. (Typically written as a percent)
relative/natural/percent abundance
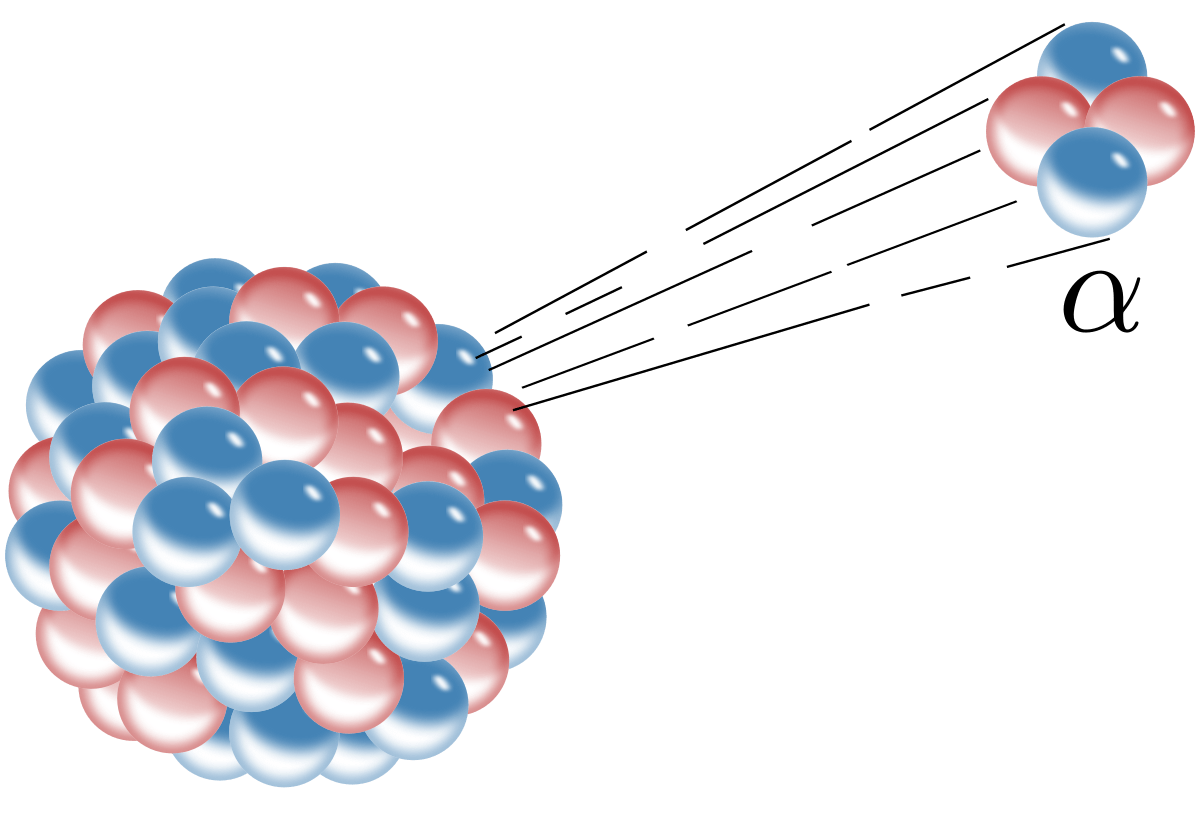
The process of emitting a He2+ ion
alpha decay
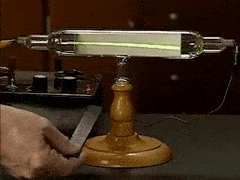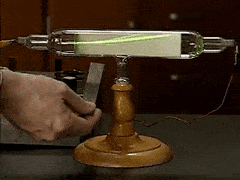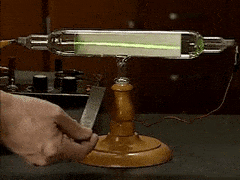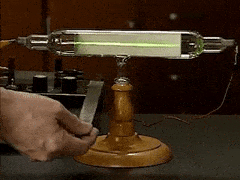
William Crookes famously discovered "negatively charged particles" using what instrument?
cathode ray tube
If an atom loses or gains electrons, then it is referred to as an ___.
ion
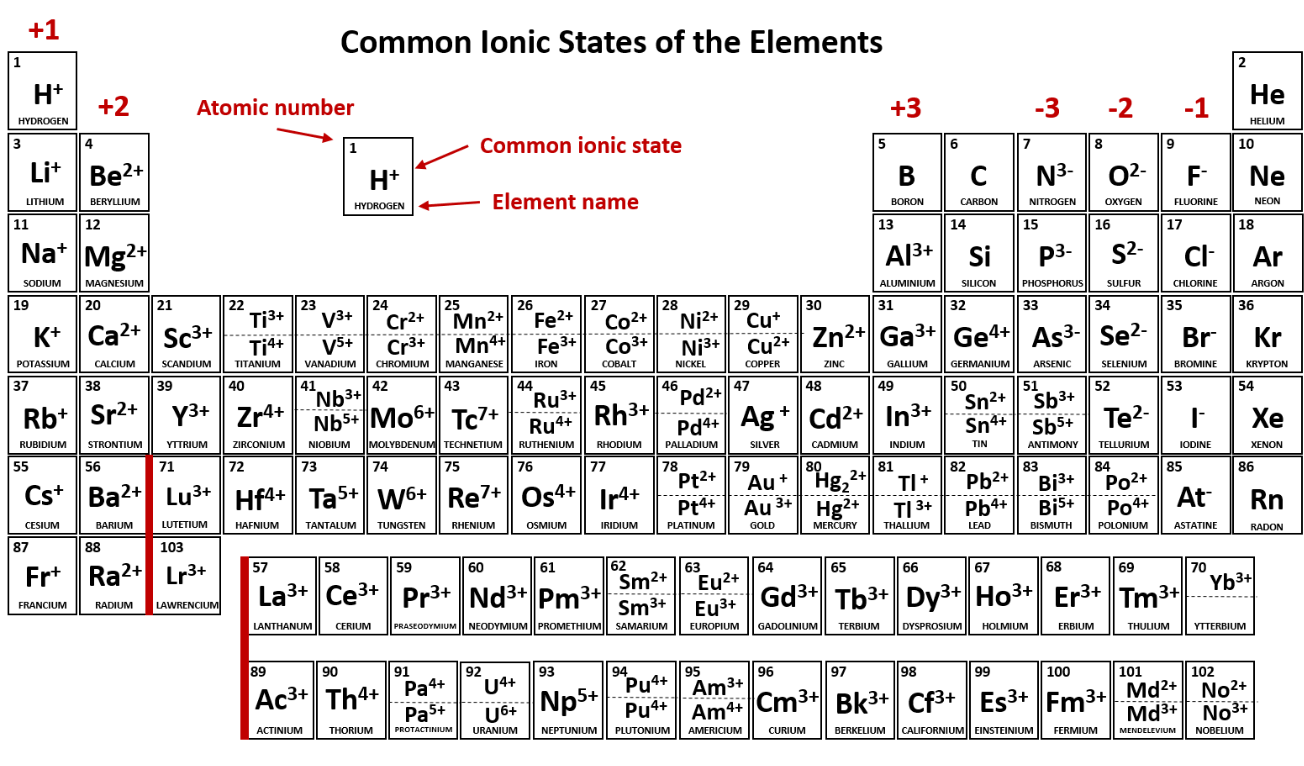
what does the number 14 represent in "nitrogen-14"?
mass number
This type of radiation is deflected toward a positively charged plate.
beta particle
What particle was used by Rutherford to investigate the structure of gold atoms.
alpha particle
The lightest subatomic particle, weighing ~2000 times less than the others.
electron
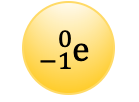
An element has two isotopes (A and B) with masses 12 and 13 amu, respectively. The average atomic mass of the element is 12.10 amu.
Which isotope of the element has the higher abundance?
isotope A
Whats happens to the nucleus of an atom after beta decay?
neutron converted to proton 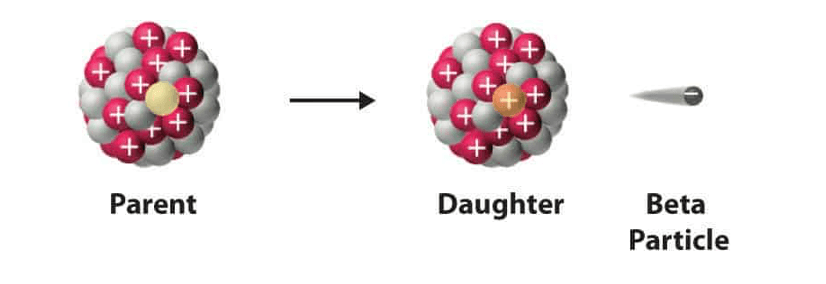
was the first to experimentally show that matter is made of what?
Dalton
Atomic number represents what?
number of protons
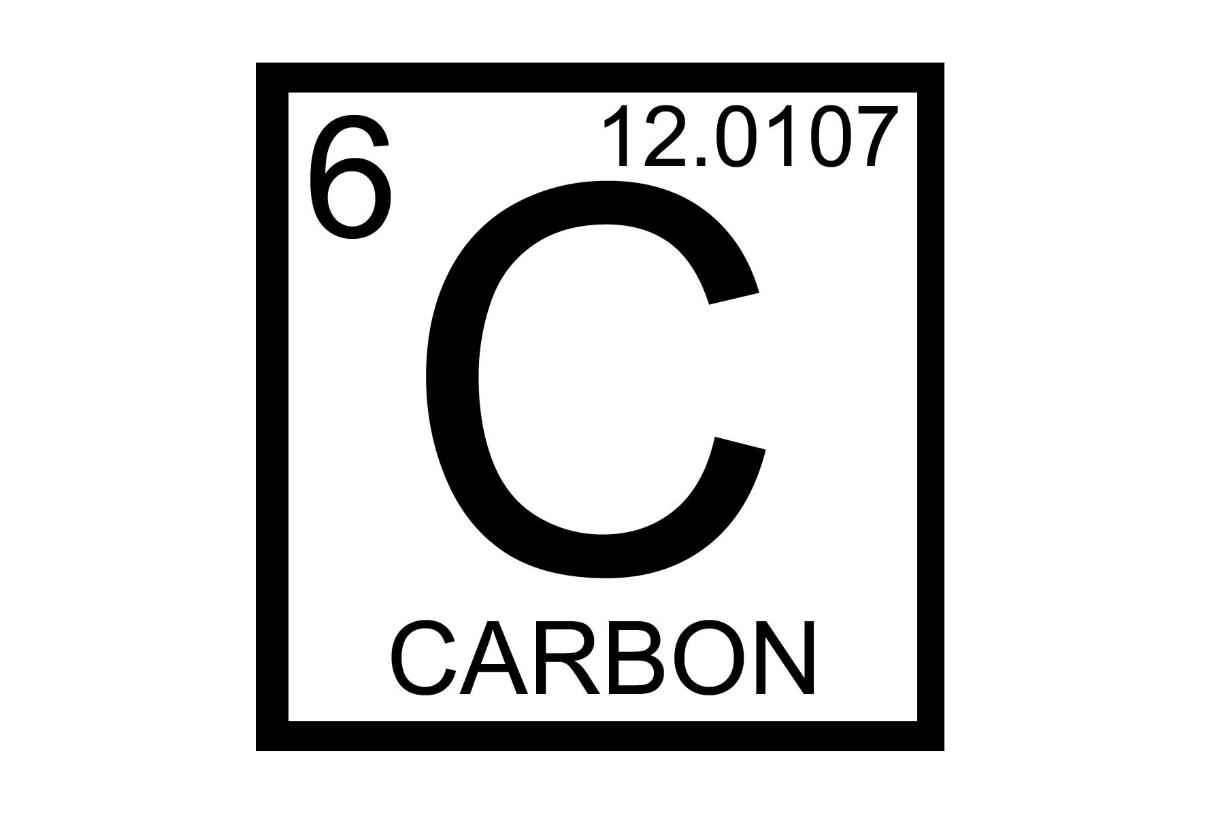
What determines the identity of an element?
#protons
Which radiation is the most harmful?
gamma radiation
Discovered the electron and proposed the obsolete plum pudding model of the atom.
J.J. Thomson
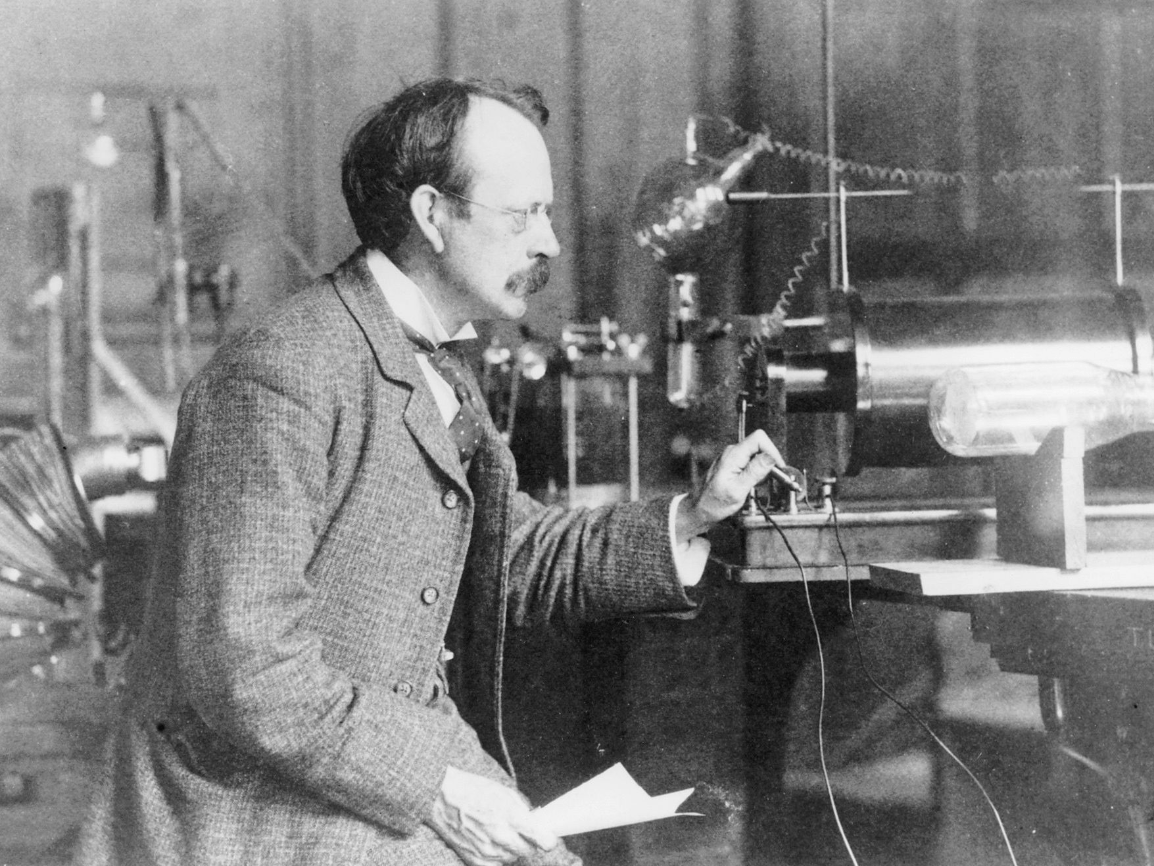
Negatively charged particles
electrons
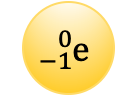
Generally, the number of protons in an atom is ___ the number of neutrons
equal to
Thin sheet of paper can block this
alpha particle
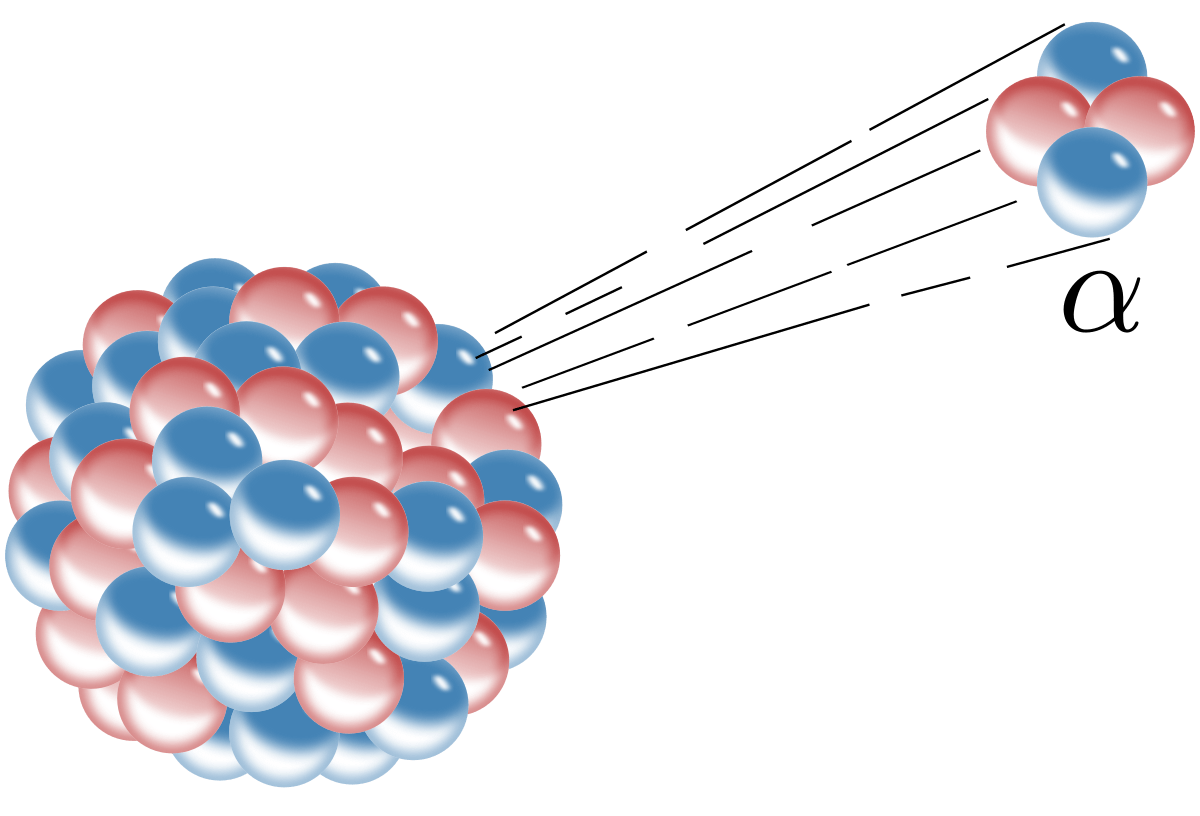
Discovered that the mass and positive charge of atoms is concentrated at the center (in the nucleus).

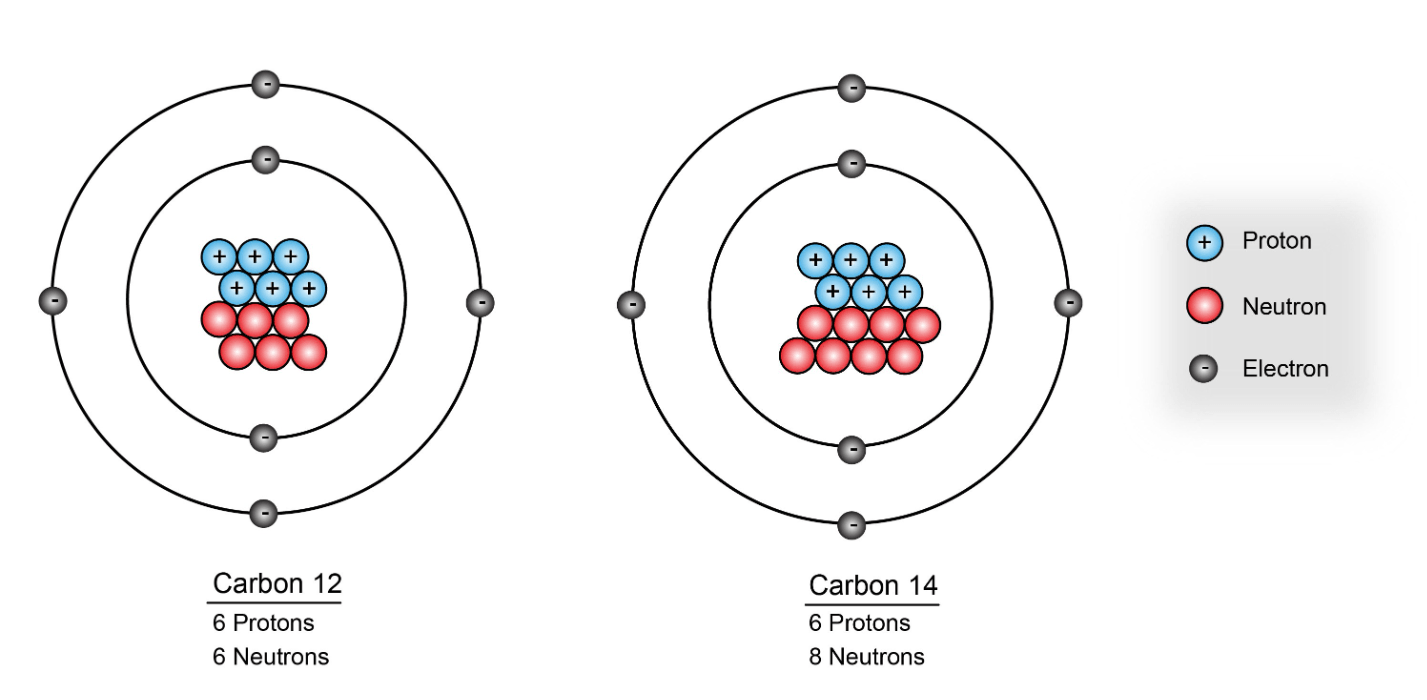
What is the mass number of carbon-12
carbon-12
mass number = 12
This subatomic particles is neutral in its charge.
neutron

At least a thin plate of aluminum is required to block this type of radioactive decay.
beta decay
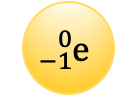
Determined the mass and charge of an electron.
Millikan

What element has an atomic number of 7
nitrogen

Too many extra neutrons will cause an atom to become __.
unstable
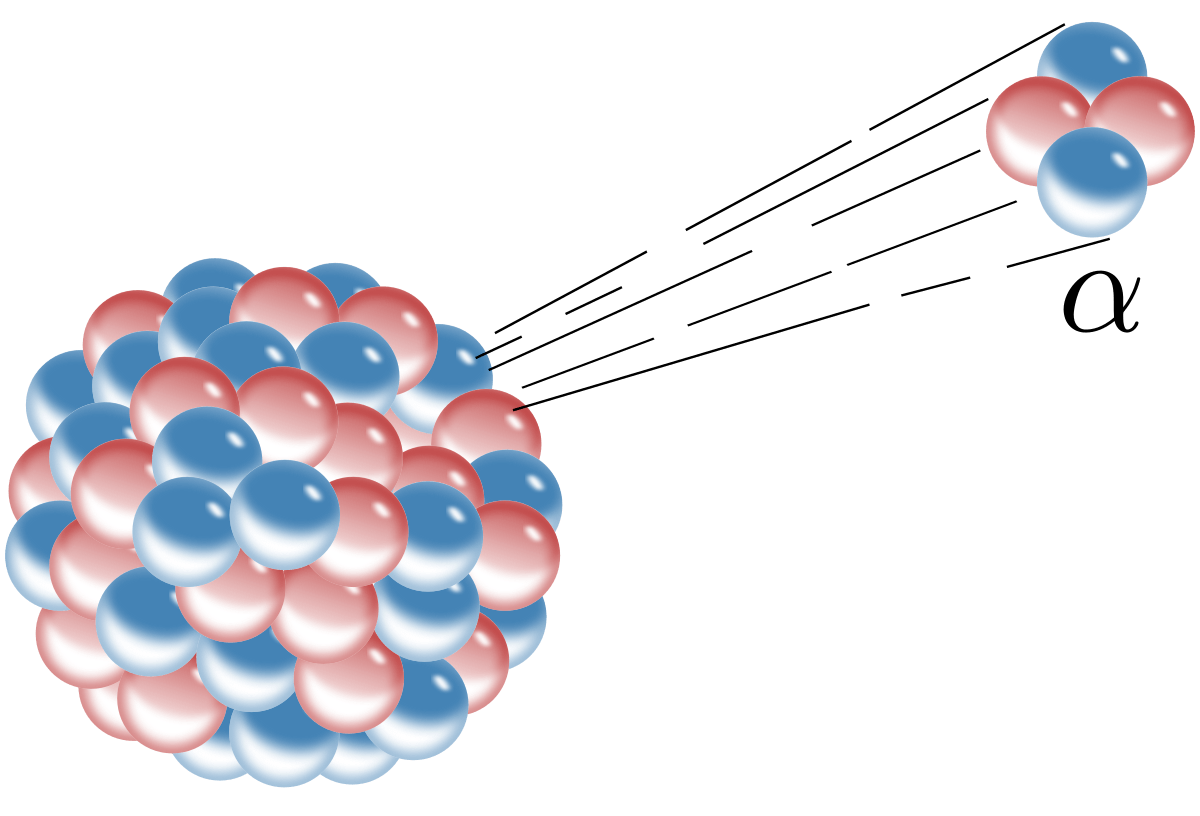
Type of radiation that is not effected by electric field from charged plates.
gamma rays

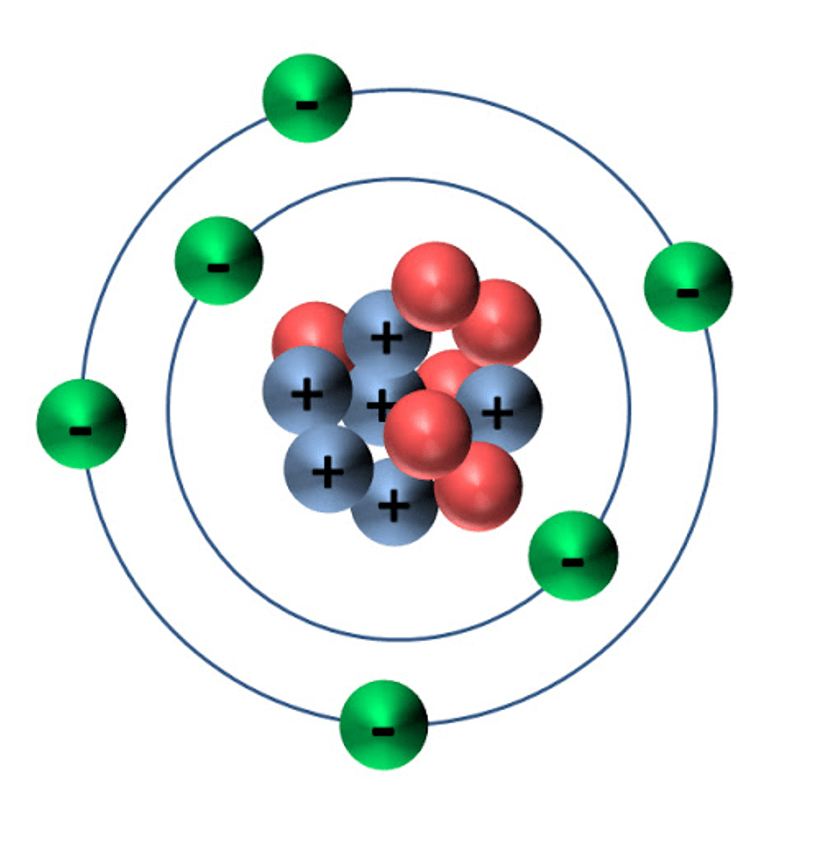
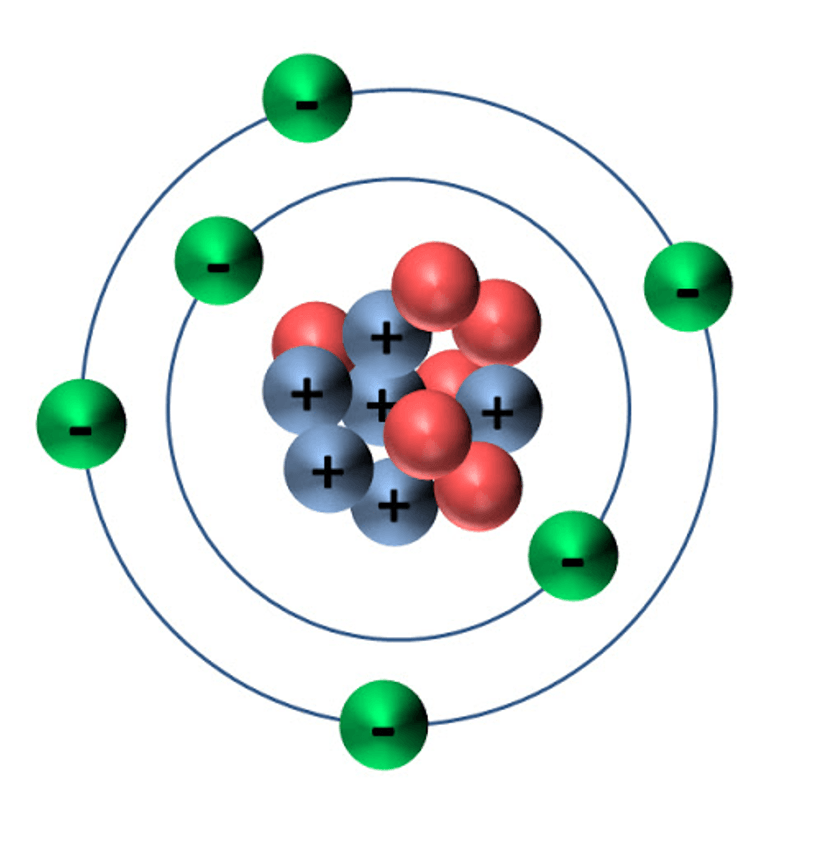
What early model of the atom is this?
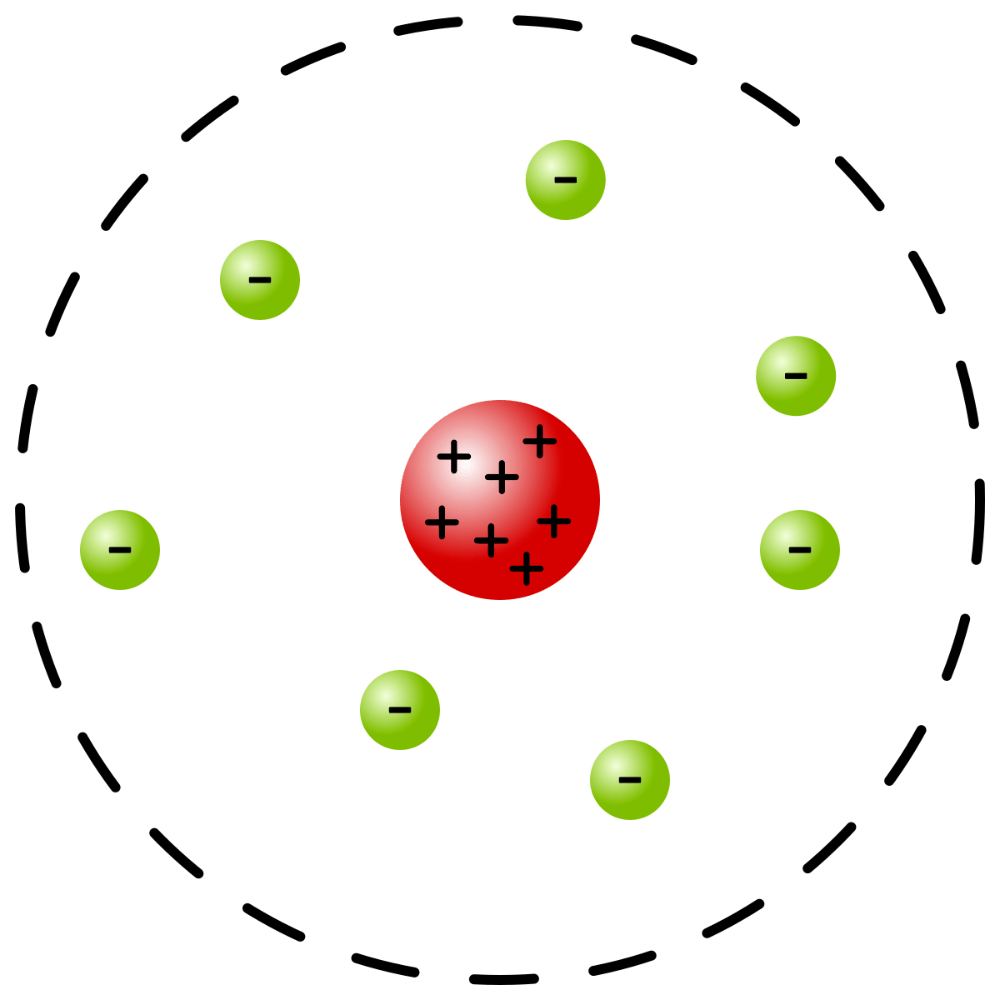
Rutherford's model

What element is this?
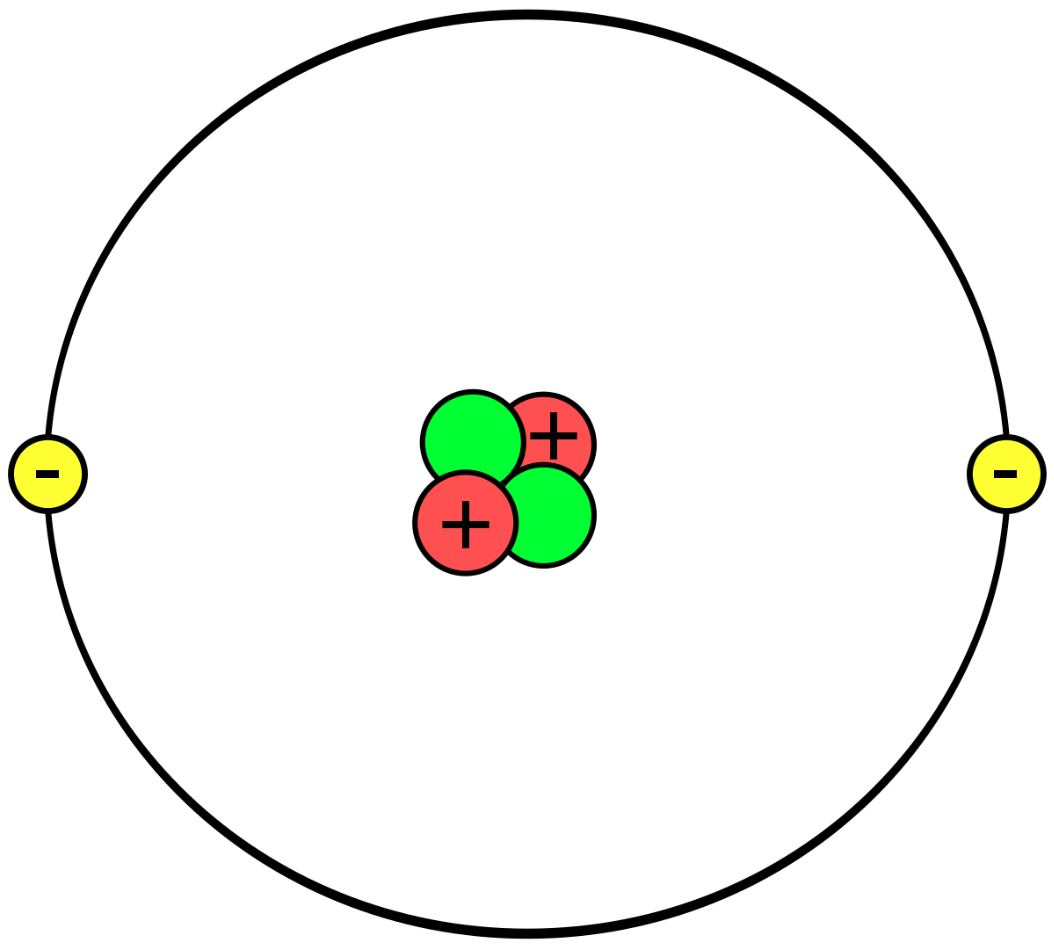
Helium
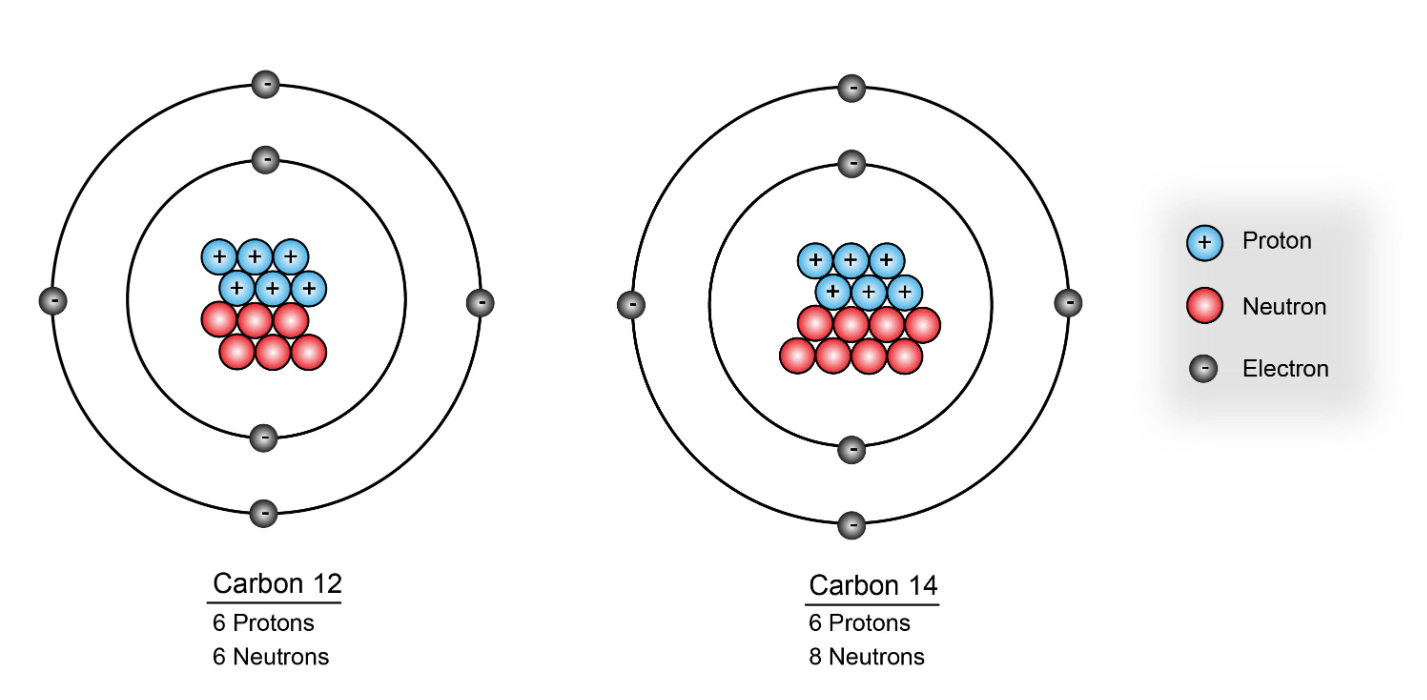
How many neutrons are in an element with 6 protons and a mass number of 14
8 neutrons
What type of radiation is produced?
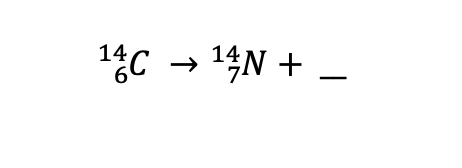
Beta particle
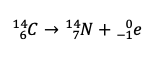
Dalton's atomic theory had 6 propositions. Which two are kn own to be incorrect today?
1. Atoms are indivisible. (nuclear decay, quarks, strings?)
2. All atoms of the same element are identical. (isotopes and ions)
How many electrons does oxygen have?
8 electrons
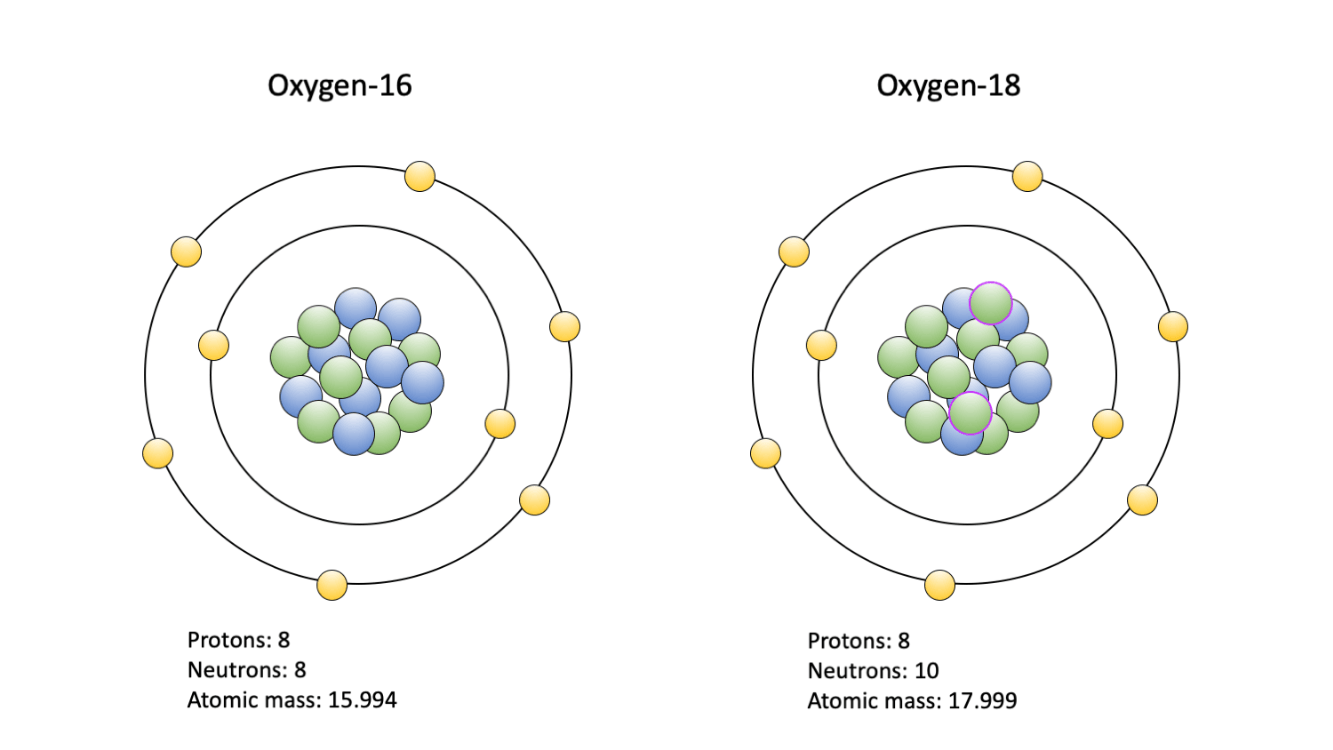
What is the average mass of of an element that has isotope A (70 amu; 50% abundance) and isotope B (72 amu; 50% abundance)?
atomic mass = [(70*50)+(72*50)]/100 = 71 amu
Uranium atom (atomic #=92) undergoes 2 consecutive alpha decays. What element is it now?
Radium (atomic #=88)