The following is an example of a ____________.
If I drink more water throughout the day, I can run further at practice.
Hypothesis
J.J. Thomson's Plum Pudding or Chocolate Chip Cookie Model was the first to introduce negatively charged _________________ to the model of an atom.
Electrons
___________ (+) and ________ (neutral) are found in the nucleus of an atom, while ____________ (-) are found in the electron cloud surrounding the nucleus.
Protons
Neutrons
Electrons
Label each statement as Element, Compound, or Mixture.
A: Two or more different elements chemically bonded
B: Made up Entirely of one type of Atom (all the same)
C: Two or more different substances in the same place but NOT chemically bonded
A: Compound
B: Element
C: Mixture
How many years did the 100 years war between England and France ACTUALLY last?
116
The _________ of Gravity explains WHY an apple falls to the ground when dropped.
Theory
True or False:
There is definitely nothing new to discover about atoms.
False
Particle 1 must be a(n) _______________ because it is found outside the Nucleus.
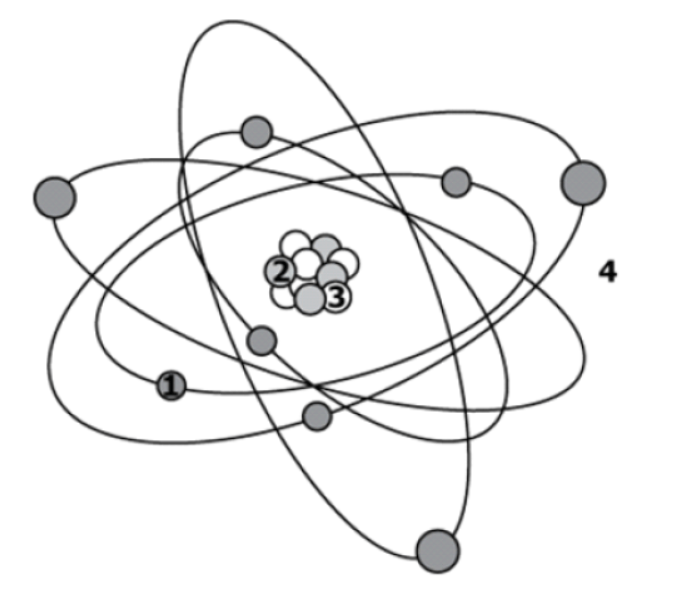
Electron
Can be broken down into a simpler substances using ONLY chemical means (chemistry).
What is a compound?
How many times has Brazil won the world cup?
5
The following is an example of a ________.
F = m*a
What is a Law
While Bohr discovered that Electron's orbit the Nucleus, what was incorrect or a limitation of his Planetary Model?
Electrons DO NOT orbit in defined paths like planets around the sun, instead they exist and orbit in a cloud like region known as the Electron Cloud
Particle 2 could either be a ___________ or a __________.
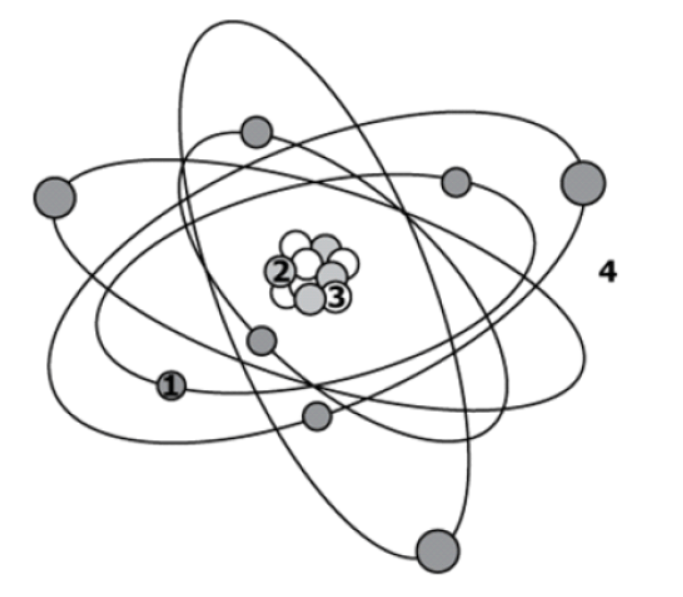
Proton or Neutron
Label each of the three images.
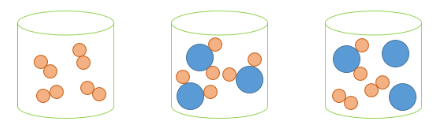
A B C
A - Element
B - Compound
C - Mixture
How many countries are in Africa?
54
A testable explanation or prediction based on observations.
True or False:
Models in science cannot include all details of the objects they represent and often must be changed or added to based on new discoveries. In other word, Models have Limitations.
True
The smallest subatomic particle (almost 2000x smaller then the others)
What is the Electron?
Can be separated into different substances using physical means. In other words, using the physical properties of the each substance to separate them.
What is a Mixture?
How many muscles are in the human body?
About 600.
An explanation of why something in the natural world happens, based on strong scientific evidence.
What is a Theory?
What discovery was made by Ernest Rutherford during his gold foil experiment where most particles pasted straight the foil?
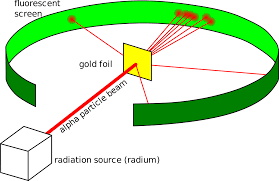
The Atom is mostly empty space with a dense nucleus at the center
These two subatomic particles have about the same mass and make up most of the atom's mass.
What are Protons and Neutrons?
Label image D and E.
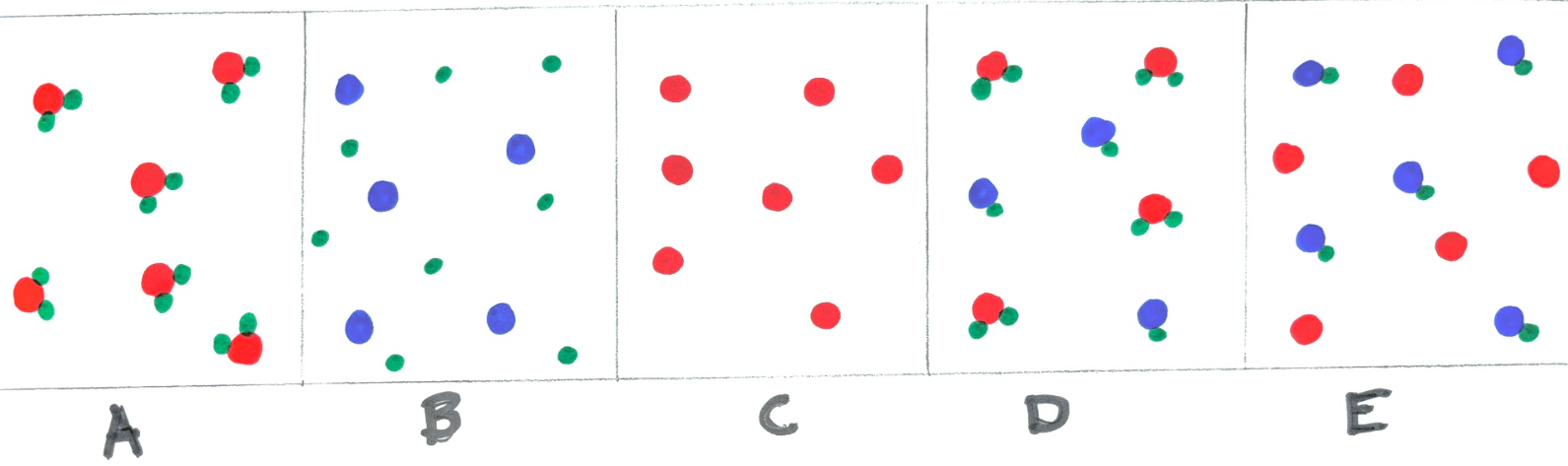
D - Mixture of Compounds
E - Mixture of Element and Compound
What is the height of Mt. Everest in meters?
8,848 meters
An explanation of what happens, in reference to something in the natural world, simple, often represented by a mathematical formula.
What is a Law?
What are two reasons that the model of the atom has changed over time, demonstrating the Nature of Science?
1. Improved Technology
2. Collaboration among scientist
3. More detailed and Specific Research
All helped lead to new discoveries
Represents the probability or likelihood of an electron's location
The Electron Cloud
Label each as either an Element, Compound, or Mixture.
a - Calcium (Ca)
b - Nitric Acid (HNO3)
c - Air
d - Silicon (Si)
e - Baking Powder (NaHCO3)
a - Element
b - Compound
c - Mixture
d - Element
e - Compound
How many hearts does a worm have?
5