Can both ionic and covalent compounds be stable?
Yes
What type of elements are used in this compound?
Metal & Nonmetal
What type of elements are used in this compound
Nonmetal & Nonmetal
What is the Lewis dot structure for NaCl?

What is IUPAC?
When you change a compounds formula into its name or vice versa.
How can a compound become stable?
An atom has to gain, lose, or share valence electrons to complete the elements outer most energy level.
What type of melting points and boiling points does this compound have?
High melting and boiling points
What type of melting and boiling points does this compound have?
Low melting and boiling points
What would the formula be?
MgCl2
What is the name for this ionic compound formula, MgI2?
Magnesium Iodide
What compound is more stable?
Ionic coumpound
What do the electrons do?
The electrons are transferred
What do the electrons do?
What type of bond is this?

Covalent bond
What is the name for this covalent compound formula, N2O3?
DinitrogenTrioxide
What is an unstable compound?
When the elements in the compound don't have a full valence electron level.
What does this compound exist as?
Crystals
What does this compound exist as?
Molecules
What bond is this?
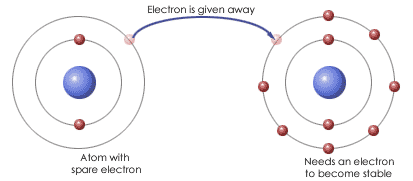
Ionic bond
What is the formula for this ionic compound name, Calcium Oxide?
CaO
What is an easy way to make a compound stable?
Lewis Dot

Which is ionic?
AL2S3 or P4S10
AL2S3
Which is covalent
BaNa2 or N2O3
N2O3

What type of bonds are these ?
HOH-Covalent
NACL-Ionic
What is the formula for this covalent compound name, TetraphosphorousDecasulfide
P4S10