These gasses reflect energy back to the Earth's surface, making it warmer.
What are greenhouse gases?
This is the name for a reaction that releases energy overall.
What is an exothermic reaction?
Balance this equation.
Nb + F --> NbF5
What is
Nb + 5F --> NbF5
This is the name of the first part of the four-stroke engine cycle.
What is intake?
This is the city that Anastasia used to live in before moving to Costa Rica.
What is Philadelphia, PA?
This is the chemical group that is typically used as a fuel in a combustion reaction.
What are hydrocarbons?
Anything that absorbs heat: An automatic ice pack, melting ice into water, boiling water into steam, cooking or baking food, etc.
This is the balanced equation for combustion of Hydrogen gas.
What is H2 + 2O2 --> 2H2O ?
This is the name for any kind of non-renewable chemical source used for energy in a vehicle.
What is "fossil fuel"?
This is the person in our class who originally owned Joaquín.
Who is J?
This is the chaos of the universe. It is always increasing.
What is entropy?
A chemical reaction releases 543 kJ of energy. What is the sign of ΔE? Is the reaction endothermic or exothermic?
ΔE is negative. Reaction is exothermic.
Fill in the blank:
S + 2H2SO4 --> __ SO2 + 2 H2O
What is
Fill in the blank:
S + 2H2SO4 --> _3_ SO2 + 2 H2O
This part of the four-stroke engine cycle converts chemical potential energy into kinetic energy.
What is the power stroke?
This is the province where Jeynor grew up as a little kid.
What is Puntarenas?
This type of energy is contained or stored in an object based on its position, chemical composition, or distance from a magnet.
What is potential energy?
ΔE for this reaction is +50 kJ/mol NH3. Put energy into the reaction correctly balanced and located.
H2 + 2NH3 --> 2NH4
H2 + 2NH3 + 100 kJ --> 2NH4
What is
2C6H6O2 + 13O2 --> 12CO2 + 6H2O
These are two fuels commonly used to power cars and two chemicals found in their exhaust.
What are diesel, gasoline, or natural gas... the two chemicals in their exhaust are carbon dioxide and water. (There are many more!)
This is the population of Costa Rica in millions of people.
What is 5 million?
This is a drawing of each of the four stages of a four-stroke cycle.

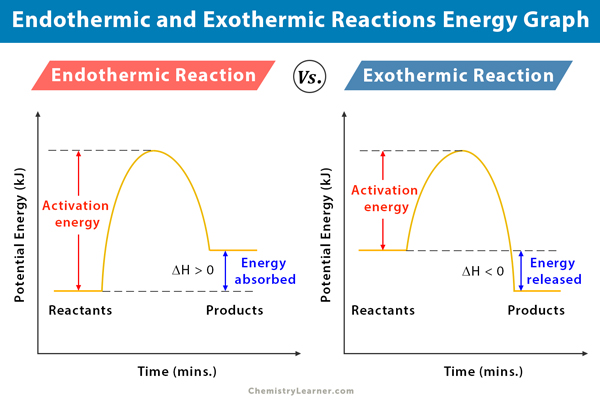
This is a graph of the potential energy in an exothermic and endothermic reaction, labeled with where bonds are broken and where bonds are made.
Balance this equation.
C2H5F + O2 --> CO2 + H2O + F2
What is
4C2H5F + 13O2 --> 8CO2 + 10H2O + 2F2
These are two reasons why Hydrogen-fueled cars are so uncommon.
Answers will vary. Includes:
-Lack of infrastructure
-Difficulty making the Hydrogen fuel in a way that is green
-Lack of efficiency in the process of making Hydrogen fuel compared with other fuel/electricity types
-Difficulty storing and transporting Hydrogen as a lightweight gas
This person famously drove a Hydrogen-cell vehicle from Liberia to San José without stopping.
Who is Franklin Chang-Díaz?