Which of the following molecules is non-polar despite having polar bonds?
A. H₂O
B. CO₂
C. NH₃
D. CHCl₃
B. CO₂ – It has polar bonds, but the molecule is linear and symmetrical, so the dipoles cancel out.
Which of the following best explains why water is a good solvent?
A. It has a high boiling point
B. It is non-polar
C. It forms hydrogen bonds and has a bent shape
D. It has a low density
C. Water’s bent shape and ability to form hydrogen bonds make it excellent at dissolving polar substances.
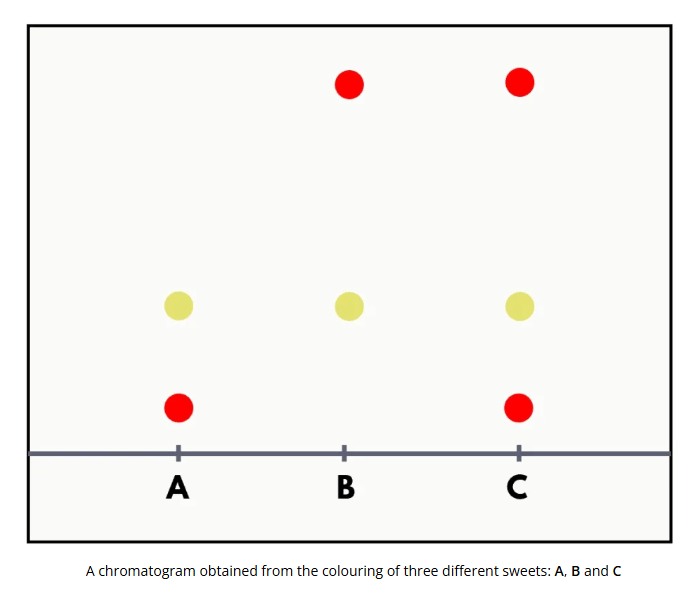
How many red dyes are there in total?
There are 2 types of red dyes.
Different compounds can give the same colour. From the chromatogram, spots at 2 different heights are red in colour, suggesting that there are two red dyes.
Are polar solutes most soluble in polar or non-polar solvents?
Polar
What is the molar volume of an ideal gas at STP?
22.7 dm3 mol-1
Draw the Lewis structure and VSEPR shape for NH₃.
Then, label the bond angles and name the molecular geometry.
- Lewis structure: N with three single bonds to H and one lone pair.
- Shape: Trigonal pyramidal
- Bond angle: ~107°

Match the following terms with their correct definitions:
- Unsaturated
- Saturated
- Supersaturated
A. Contains more solute than normally possible at a given temperature
B. Contains less solute than it can hold at a given temperature
C. Contains the maximum amount of solute at a given temperature
- 1 → B
- 2 → C
- 3 → A
Using your formula and data book, predict whether a precipitate will form when BaCl₂ is mixed with Na₂SO₄.
Write the balanced ionic equation including states.
What name is given to a solution which has dissolved in it more solute than normally possible at a given temperature?
Supersaturated
How does increasing the temperature of the system increase the rate of reaction?
Increasing the temperature of the system will increase the proportion of particles that possess an average kinetic energy greater than or equal to the activation energy of the reaction, thereby increasing the number of colliding particles with enough energy to react leading to more fruitful collisions, thereby increasing the rate of reaction.
Rank the following substances from lowest to highest boiling point, based on the dominant intermolecular forces:
- CH₄
- H₂O
- CH₃Cl
- C6H14
Lowest → Highest boiling point:
CH₄ (dispersion) <C6H14 (dispersion) < CH₃Cl (dipole-dipole) < H₂O (hydrogen bonding)
Draw a water molecule showing its shape, bond angles, and partial charges.
Then, explain how this contributes to hydrogen bonding.
- Shape: Bent
- Bond angle: ~104.5°
- Partial charges: δ⁻ on oxygen, δ⁺ on hydrogens
- Explanation: The polarity and bent shape allow water molecules to attract each other via hydrogen bonds.

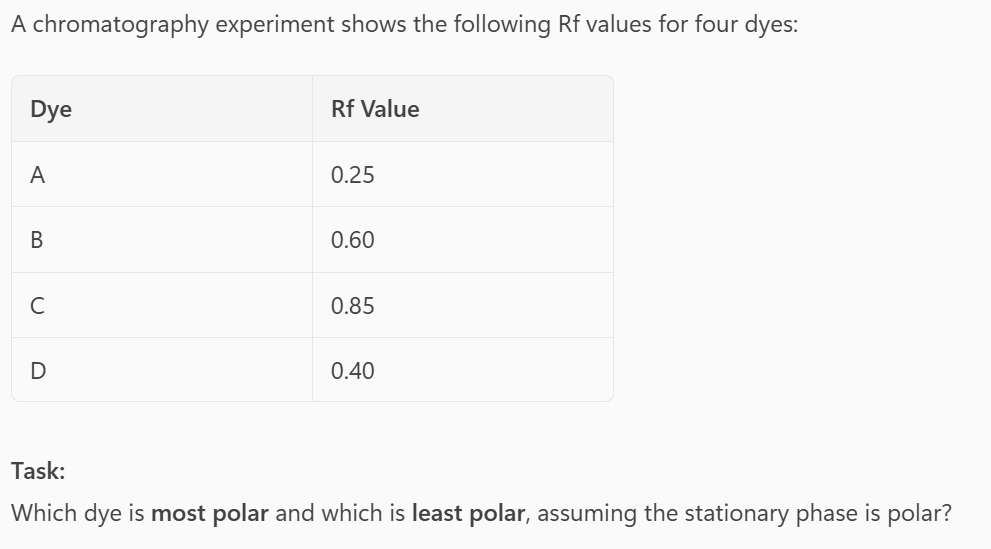
- Most polar → Dye A (lowest Rf, sticks to polar stationary phase)
- Least polar → Dye C (highest Rf, moves with non-polar mobile phase)
When dissolving a solute in a solvent, does increasing a solvent’s temperature increase or decrease the solubility?
It depends on the solute. It increases the solubility if the solute is a solid; decreases solubility if the solvent is a gas.
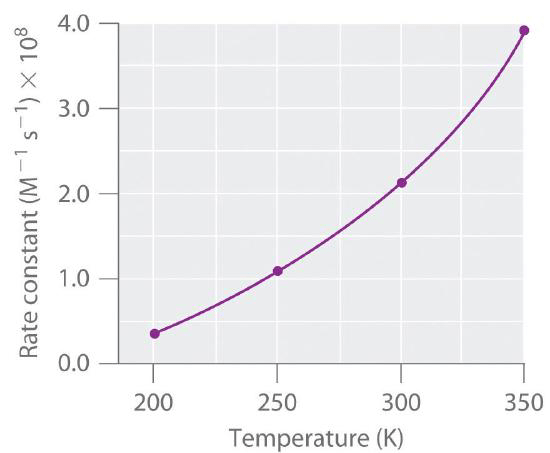
This graph represents the change in a rate constant for a forward reaction under a change in temperature. Is the favoured reaction an exothermic or endothermic reaction?
Le Chatelier’s principle states that, for an endothermic reaction, an increase in temperature will cause the system to work to decrease the temperature, by favouring an endothermic reaction direction. Therefore, the favoured reaction is endothermic, and its rate will increase.
***********************DAILY DOUBLE***********************
....
....
.....
....
....
Explain why water has a much higher boiling point than methane, even though both are small molecules.
Water has hydrogen bonding, a strong intermolecular force due to the highly electronegative oxygen bonded to hydrogen. Methane only has dispersion forces, which are much weaker. This means more energy is needed to break the intermolecular forces in water, resulting in a higher boiling point.
Explain the difference between strength and concentration in terms of acids and bases.
Give an example of a strong but dilute acid.
- Strength refers to the degree of ionisation (e.g. strong acids fully ionise).
- Concentration refers to the amount of solute in a given volume.
- Example: 0.01 M HCl is strong (fully ionises) but dilute (low concentration).
A solubility curve shows that 40 g of KNO₃ dissolves in 100 mL of water at 30°C, and 60 g dissolves at 50°C.
Task:
If a student dissolves 50 g of KNO₃ in 100 mL of water at 30°C, is the solution unsaturated, saturated, or supersaturated?
Supersaturated – 50 g > 40 g at 30°C
What are the units of solubility?
Accept any ratio of solute to solvent (e.g. molarity, molality, grams solute/100g solvent, mass/volume, etc.)
2NO2 (brown gas) and N2O4 (colourless gas) exist in equilibrium. Increasing the pressure of the system will favour which colour?
According to Le Chatelier’s principle, increasing the pressure will cause the system to work to decrease the pressure by favouring the reaction with the least amount of molecules produced. Therefore the system will produce more N2O4 and become colourless.
You are given three unknown substances:
- Substance A evaporates quickly and has a low boiling point.
- Substance B is a liquid at room temperature and dissolves well in water.
Task:
Match each substance to the likely dominant intermolecular force:
- Dispersion forces
- Dipole-dipole attractions
- Hydrogen bonding
- A → Dispersion forces
- B → Hydrogen bonding
You have 0.5 moles of NaCl dissolved in 250 mL of water.
Calculate the concentration of the solution in mol/L.

You perform a series of tests on an unknown solution:
- Adding HCl produces bubbles.
- Adding BaCl₂ produces a white precipitate.
Task:
Identify one ion that might be present. Provide 2 pieces of evidence.
Carbonate - Acid + carbonate produces carbon dioxide (bubbles)
Carbonate and barium is insoluble.
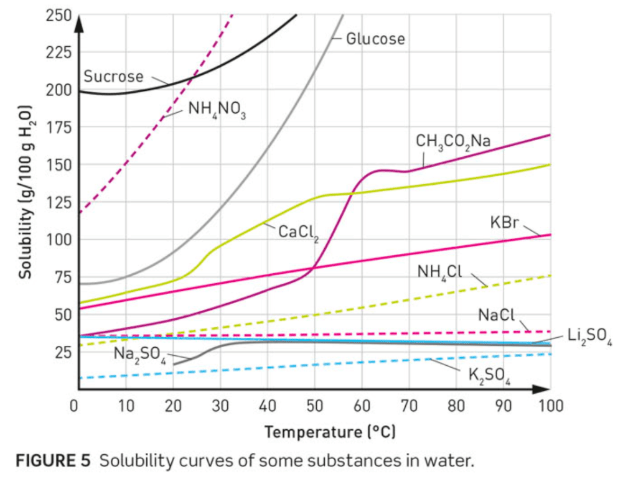
There is 300g of CaCl2 dissolved in 300g of water at 60 oC. Is it saturated?
No.
That is equivalent in concentration to 100g CaCl2 in 100g water, which is below the solubility curve.
Calculate the volume (in Litres) of a gaseous system of carbon dioxide that is at 27kPa, and at 42ºC when 856g of carbon dioxide are present.
1,887 Litres.