This subatomic particle has a positive charge.
What is a proton?
He discovered the electron using the cathode ray tube.
Who is J.J. Thomson?
The first energy level can hold this many electrons.
What is 2?
The dots in a Lewis structure represent these particles.
What are valence e-'s?
Which university did Microsoft founder Bill Gates drop out of?
Harvard
The negatively charged particle that orbits the nucleus.
What is an electron?
He proposed that atoms are solid spheres that cannot be divided.
Who is John Dalton?
The second energy level can hold this many electrons.
What is 8?
These element’s Lewis dot diagram shows only one dot.
What is group 1?
What year was the first iPhone released?
2007
Most of the atom’s mass is found in this region.
What is the nucleus?
He discovered the nucleus through the gold foil experiment.
Who is Ernest Rutherford?
In a Bohr model of oxygen, how many energy levels are needed?
What is 2?
How many dots are around oxygen in its Lewis dot diagram?
What is 6?
In the car industry, what does "BMW" stand for?
Bavarian Motor Works
This particle determines the atomic number of an element.
What is the proton?
This scientist proposed that electrons orbit the nucleus in specific energy levels.
Who is Niels Bohr?
A neutral atom has the same number of these two subatomic particles.
What is protons and electrons?
All the elements in group 18 have 8 valence electrons except this element.
What is helium?
Which country consumes the most chocolate per capita?
Switzerland
Isotopes of the same element differ in the number of these particles.
What are neutrons?
This model describes electrons as existing in a “cloud” rather than fixed orbits.
What is the quantum mechanical model (or electron cloud model)?
This is the Bohr model of _____________
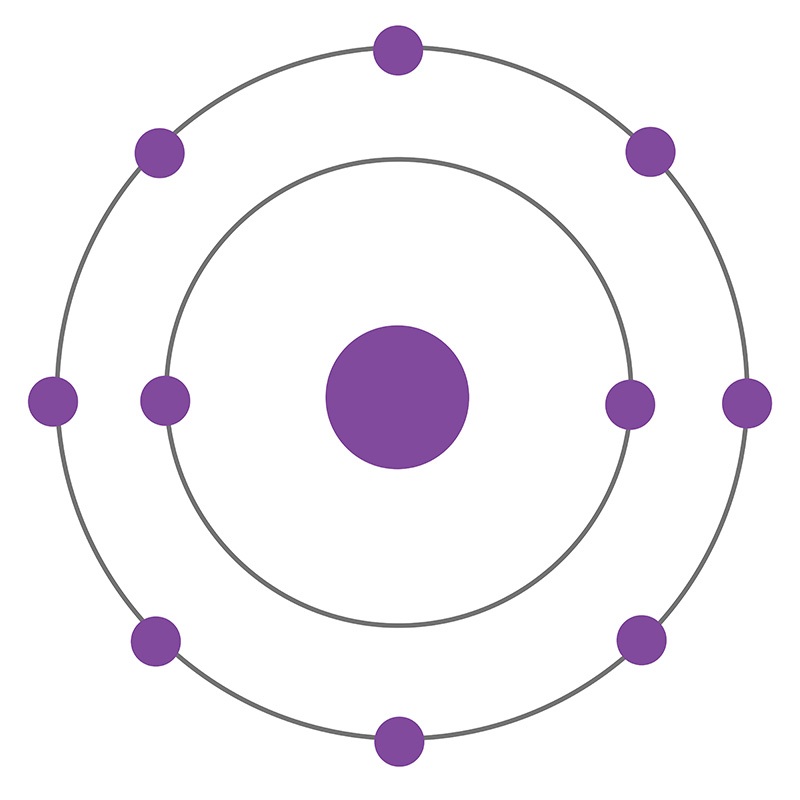
What is neon?
What is the difference between a Bohr Model and Lewis Dot Structure?
Lewis only shows the ve-
What is the national animal of Scotland?
The Unicorn